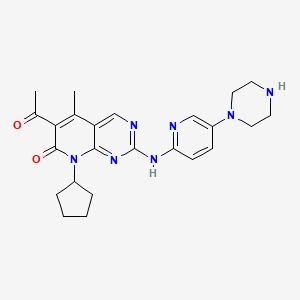
Palbociclib
概要
説明
パロブシクリブは、ファイザーが開発したホルモン受容体陽性およびヒト上皮成長因子受容体2陰性の乳がんの治療薬です . これは、細胞周期調節において重要な役割を果たすサイクリン依存性キナーゼ4と6の選択的阻害剤です . パロブシクリブは、がん治療薬として承認された最初のサイクリン依存性キナーゼ4および6阻害剤でした .
製造方法
パロブシクリブの製造には、いくつかの合成経路と反応条件が含まれています。 1つの方法には、以下の手順が含まれます :
環化反応: 2-アセチル-2-ブテン酸メチルエステルとマロンニトリルはアルカリ条件下で反応して、1,4,5,6-テトラヒドロ-2-メトキシル-4-メチル-5-アセチル-6-オキシ-3-ピリジンカルボニトリルを生成します。
置換反応: 中間生成物は、酸結合剤の影響下でハロゲン化シクロペンタンと反応して、N-シクロペンチル-1,4,5,6-テトラヒドロ-2-メトキシル-4-メチル-5-アセチル-6-オキシ-3-ピリジンカルボニトリルを生成します。
縮合反応: 中間生成物は、N-[5-(1-ピペラジニル)-2-ピリジニル]グアニジンと反応して、6-アセチル-8-シクロペンチル-5,8-ジヒドロ-5-メチル-2-[5-(1-ピペラジニル)-2-ピリジニル]アミノ-ピリド[2,3-d]ピリミジン-7(6H)-オンを生成します。
脱水素反応: 中間生成物はセレン酸ナトリウムと反応して、パロブシクリブを調製します。
作用機序
生化学分析
Biochemical Properties
Palbociclib acts in the cell cycle machinery . It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6 . CDK4 and CDK6 form complexes with cyclin D to promote phosphorylation of the retinoblastoma (Rb) protein, which allows cell cycle progression .
Cellular Effects
This compound inhibits cell viability and blocks cell cycle at the G1 phase, inducing cell senescence . It also inhibits migration and invasion in certain cancer cells . In breast cancer cells, this compound works with hormonal therapy drugs to slow the cancer’s growth and spread .
Molecular Mechanism
This compound is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6 . It blocks the transition from the G1 to the S phase by binding to CDK4/6, inhibiting Rb protein phosphorylation . This prevents the cell from passing the restriction point and exiting G1, thereby halting the cell cycle .
Temporal Effects in Laboratory Settings
This compound has shown significant inhibitory effects in various tumor models in vivo . It has been observed that a short exposure of cells to this compound is sufficient to produce a stable cell-cycle arrest and long-term senescence . After washing out the drug, this compound-treated cells release the drug to the medium, which can induce senescence in susceptible cells .
Dosage Effects in Animal Models
In animal models, this compound has shown significant inhibitory effects on tumor growth at various dosages . The effects of this compound vary with different dosages, with substantial reductions in total tumor volumes and in Ki-67 proliferation marker expression observed .
Metabolic Pathways
This compound is mainly metabolized in the liver via oxidation and sulfonation, primarily by the cytochrome P450 isoenzyme 3A and the sulfotransferase 2A1 . Acylation and glucuronidation are minor metabolic pathways .
Transport and Distribution
This compound concentrates in intracellular acidic vesicles, where it can be readily observed due to its intrinsic fluorescence . It is released from these vesicles upon dilution or washing out of the extracellular medium .
Subcellular Localization
This compound is stored in acidic vesicles within the cell . This lysosomal trapping of this compound explains the prolonged temporal activity of the drug, its paracrine activity, and its cooperation with other lysosomotropic drugs .
準備方法
The preparation of Palbociclib involves several synthetic routes and reaction conditions. One method includes the following steps :
Ring-closing reaction: 2-acetyl-2-butenoic acid methyl ester and malononitrile react under alkaline conditions to generate 1,4,5,6-tetrahydro-2-methoxyl-4-methyl-5-acetyl-6-oxy-3-pyridine carbonitrile.
Substitution reaction: The intermediate product reacts with halogenated cyclopentane under the effect of an acid-binding agent to generate N-cyclopentyl-1,4,5,6-tetrahydro-2-methoxyl-4-methyl-5-acetyl-6-oxy-3-pyridinecarbonitrile.
Condensation reaction: The intermediate product reacts with N-[5-(1-piperazinyl)-2-pyridinyl]guanidine to generate 6-acetyl-8-cyclopentyl-5,8-dihydro-5-methyl-2-[5-(1-piperazinyl)-2-pyridinyl]amino-pyrido[2,3-d]pyrimidin-7(6H)-one.
Dehydrogenation reaction: The intermediate product reacts with sodium selenate to prepare this compound.
This method is economical, environmentally friendly, and suitable for industrial production .
化学反応の分析
パロブシクリブは、置換反応、酸化還元反応、縮合反応など、さまざまな種類の化学反応を起こします . これらの反応で使用される一般的な試薬には、塩化チオニル、臭素、シクロペンチルアミン、セレン酸ナトリウムが含まれます . これらの反応から生成される主な生成物には、1,4,5,6-テトラヒドロ-2-メトキシル-4-メチル-5-アセチル-6-オキシ-3-ピリジンカルボニトリルや6-アセチル-8-シクロペンチル-5,8-ジヒドロ-5-メチル-2-[5-(1-ピペラジニル)-2-ピリジニル]アミノ-ピリド[2,3-d]ピリミジン-7(6H)-オンなどの中間体が含まれます .
科学研究への応用
パロブシクリブは、化学、生物学、医学、産業の分野で特に幅広い科学研究への応用があります . 医学では、主にホルモン受容体陽性およびヒト上皮成長因子受容体2陰性の進行性または転移性乳がんの治療に使用されます . さらに、パロブシクリブは、c-Myc G-四重鎖構造を安定化させる能力が研究されており、これはがん治療に影響を与える可能性があります .
科学的研究の応用
Palbociclib has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry . In medicine, it is primarily used to treat hormone receptor-positive and human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer . Additionally, this compound has been studied for its ability to stabilize c-Myc G-quadruplex structures, which could have implications for cancer treatment .
類似化合物との比較
特性
IUPAC Name |
6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AHJRHEGDXFFMBM-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C(=O)N(C2=NC(=NC=C12)NC3=NC=C(C=C3)N4CCNCC4)C5CCCC5)C(=O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C24H29N7O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40972590 | |
| Record name | Palbociclib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40972590 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
447.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Palbociclib is a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor that acts by binding to the ATP pocket with an IC50 in the range of 9-15 nmol/L. It is important to consider that it presents low to absent activity against other kinases. The CDK4/6 kinase is involved, with coregulatory partner cyclin D, in the G1-S transition. Hence, inhibition of this step prevents cell cycle progression in cells in whose this pathway is functioning. This step includes the pathways of the phosphorylation of retinoblastoma protein and the E2F family of transcription factors. | |
| Record name | Palbociclib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
571190-30-2 | |
| Record name | Palbociclib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=571190-30-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Palbociclib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0571190302 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Palbociclib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Palbociclib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40972590 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PALBOCICLIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/G9ZF61LE7G | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
263-266 ºC | |
| Record name | Palbociclib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。