トリクラベンダゾール
概要
説明
トリクラベンダゾールは、主に肝臓吸虫(特にFasciola hepaticaとFasciola gigantica)による感染症の治療に用いられるベンゾイミダゾール誘導体です . エガテンやファシネックスなどの商品名で販売されています . トリクラベンダゾールは、幼虫期と成虫期の両方の肝臓吸虫に対して効果を発揮するという点で、他のベンゾイミダゾール系薬剤とは異なります .
製造方法
合成経路と反応条件
トリクラベンダゾールは、さまざまな方法で合成できます。 一般的な方法の一つは、1,2,3-トリクロロベンゼンを出発物質とし、高濃度のアルカリ液中で加水分解して2,3-ジクロロフェノールナトリウムを生成することです . この中間体は、メチルベンゼン水溶液中で4,5-ジクロロ-2-ニトロアニリンと反応して、4-クロロ-5-(2,3-ジクロロフェノキシ)-2-ニトロアニリンを生成します . 次に、水素触媒移動法を用いてニトロ基を還元し、得られた化合物をメチル化してトリクラベンダゾールを製造します .
別の方法では、3,4-ジクロロアニリンを出発物質として、アシル化、ニトロ化、加水分解、縮合、ヒドラジン水和物による還元、S-メチルイソチオ尿素硫酸塩による環化を行う方法があります . この方法は、危険な試薬や高圧反応を使用しないため、より安全で環境に優しい方法です .
工業生産方法
トリクラベンダゾールの工業生産では、通常、上記のような合成経路に従い、大規模製造に最適化されます。 安価で入手しやすい出発物質と環境に優しい試薬を使用することで、コスト効率の高い大規模生産に適したプロセスとなっています .
科学的研究の応用
Triclabendazole has a wide range of scientific research applications:
Chemistry: Used as a model compound to study benzimidazole derivatives and their chemical properties.
Biology: Investigated for its effects on liver flukes and other parasitic organisms.
Medicine: Primarily used to treat fascioliasis and paragonimiasis in humans and animals It is the only FDA-approved drug for fascioliasis in humans.
Industry: Used in veterinary medicine to treat liver fluke infections in livestock.
作用機序
トリクラベンダゾールとその代謝物は、肝臓吸虫の被膜に吸収され、安静膜電位の低下と運動性の阻害につながります . この障害は、吸虫の表面と超微細構造に影響を与え、最終的にその死滅につながります . トリクラベンダゾールは、ベータチューブリンに結合して、微小管の重合を阻害します。微小管は、吸虫の生存に不可欠です .
類似の化合物との比較
トリクラベンダゾールは、幼虫期と成虫期の両方の肝臓吸虫に対して効果を発揮するという点で、他のベンゾイミダゾール系薬剤とは異なります . 類似の化合物には以下のようなものがあります。
アルベンダゾール: さまざまな寄生虫感染症の治療に用いられますが、肝臓吸虫に対する効果は低いです.
チアベンダゾール: 別のベンゾイミダゾール誘導体であり、作用機序が異なります。主にストロンジロイディアシスの治療に用いられます.
トリクラベンダゾールの独特の構造には、塩素化ベンゼン環とカルバメート基の欠如が含まれ、その独特の作用機序と効果に貢献しています .
生化学分析
Biochemical Properties
Triclabendazole and its metabolites are active against both the immature and mature worms of Fasciola hepatica and Fasciola gigantica helminths . It is mainly metabolized by the CYP1A2 enzyme into its active sulfoxide metabolite and to a lesser extent by CYP2C9, CYP2C19, CYP2D6, CYP3A, and FMO (flavin containing monooxygenase) .
Cellular Effects
Triclabendazole has been found to induce lytic cell death in MCF-7 and MDA-MB-231 breast cancer cells, a typical sign of pyroptosis . It activates apoptosis by regulating the apoptotic protein levels including Bax, Bcl-2, and enhanced cleavage of caspase-8/9/3/7 and PARP . In addition, enhanced cleavage of GSDME was also observed, which indicates the secondary necrosis/pyroptosis is further induced by active caspase-3 .
Molecular Mechanism
The molecular mode of action of triclabendazole consists in binding to beta-tubulin, therefore preventing the polymerization of microtubules . This disrupts the structural integrity of the helminths, leading to their death .
Temporal Effects in Laboratory Settings
In a study on 350 individuals with metabolic syndrome high-risk, after a 3-month proactive intervention, two-thirds of the phenotypic markers were significantly improved in the cohort . This suggests that triclabendazole has a time-dependent effect on biochemical markers.
Dosage Effects in Animal Models
In veterinary medicine, triclabendazole is typically administered at an oral dose of 10 or 12 mg/kg body weight to sheep and cattle, respectively . The effects of triclabendazole vary with different dosages in animal models. For example, in a study on sheep naturally infected with Fasciola sp., treatment with triclabendazole resulted in significant reduction in fecal egg count .
Metabolic Pathways
Triclabendazole is metabolized within the host, principally into its sulphoxide and sulphone metabolites . This biotransformation is carried out by the flavin monooxygenase (FMO) and cytochrome P450 (CYP 450) enzyme systems .
Transport and Distribution
Triclabendazole and its metabolites are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential . This suggests that triclabendazole is transported and distributed within cells and tissues via absorption.
Subcellular Localization
Given its mechanism of action, it is likely that triclabendazole and its metabolites localize to regions where beta-tubulin is abundant, such as the cytoskeleton of cells .
準備方法
Synthetic Routes and Reaction Conditions
Triclabendazole can be synthesized using various methods. One common method involves starting with 1,2,3-trichlorobenzene, which undergoes hydrolysis in high-concentration alkali liquor to form 2,3-dichlorophenol sodium . This intermediate reacts with 4,5-dichloro-2-nitroaniline in a methylbenzene aqueous solution to form 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline . The nitro group is then reduced using a hydrogen catalytic transfer method, and the resulting compound undergoes methylation to yield triclabendazole .
Another method involves using 3,4-dichloroaniline as the starting material, followed by acylation, nitration, hydrolysis, condensation, reduction with hydrazine hydrate, and ring-closure with S-methylisothiourea sulfate . This method avoids the use of hazardous reagents and high-pressure reactions, making it safer and more environmentally friendly .
Industrial Production Methods
Industrial production of triclabendazole typically follows the synthetic routes mentioned above, with optimizations for large-scale manufacturing. The use of inexpensive and readily available starting materials, along with environmentally friendly reagents, makes the process cost-effective and suitable for large-scale production .
化学反応の分析
反応の種類
トリクラベンダゾールは、さまざまな化学反応を起こします。これには以下のような反応が含まれます。
一般的な試薬と条件
生成される主な生成物
スルホンとスルホキシド代謝物: トリクラベンダゾールが肝臓で酸化されて生成されます.
4-クロロ-5-(2,3-ジクロロフェノキシ)-2-ニトロアニリン: トリクラベンダゾールの合成における中間体です.
科学研究への応用
トリクラベンダゾールは、さまざまな科学研究に応用されています。
類似化合物との比較
Triclabendazole is unique among benzimidazoles due to its efficacy against both immature and mature liver flukes . Similar compounds include:
Albendazole: Used to treat a variety of parasitic infections but less effective against liver flukes.
Thiabendazole: Another benzimidazole derivative with a different mechanism of action, primarily used to treat strongyloidiasis.
Closantel: Effective against immature liver flukes but not as broad-spectrum as triclabendazole.
Triclabendazole’s unique structure, including a chlorinated benzene ring and the absence of a carbamate group, contributes to its distinct mechanism of action and efficacy .
特性
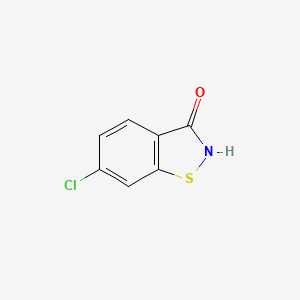
IUPAC Name |
6-chloro-5-(2,3-dichlorophenoxy)-2-methylsulfanyl-1H-benzimidazole | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H9Cl3N2OS/c1-21-14-18-9-5-8(16)12(6-10(9)19-14)20-11-4-2-3-7(15)13(11)17/h2-6H,1H3,(H,18,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NQPDXQQQCQDHHW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CSC1=NC2=CC(=C(C=C2N1)Cl)OC3=C(C(=CC=C3)Cl)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H9Cl3N2OS | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7043952 | |
| Record name | Triclabendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7043952 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
359.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
0.5 [ug/mL] (The mean of the results at pH 7.4) | |
| Record name | SID50085431 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Triclabendazole is an anthelmintic agent against _Fasciola_ species. The mechanism of action against Fasciola species is not fully understood at this time. In vitro studies and animal studies suggest that triclabendazole and its active metabolites (_sulfoxide_ and _sulfone_) are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential, the inhibition of tubulin function as well as protein and enzyme synthesis necessary for survival. These metabolic disturbances lead to an inhibition of motility, disruption of the worm outer surface, in addition to the inhibition of spermatogenesis and egg/embryonic cells. **A note on resistance** In vitro studies, in vivo studies, as well as case reports suggest a possibility for the development of resistance to triclabendazole. The mechanism of resistance may be multifactorial and include changes in drug uptake/efflux mechanisms, target molecules, and changes in drug metabolism. The clinical significance of triclabendazole resistance in humans is not yet elucidated. | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
68786-66-3 | |
| Record name | Triclabendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=68786-66-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Triclabendazole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0068786663 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Triclabendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759250 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Triclabendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7043952 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1H-Benzimidazole, 6-chloro-5-(2,3-dichlorophenoxy)-2-(methylthio) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.414 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRICLABENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4784C8E03O | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
189-191 | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。