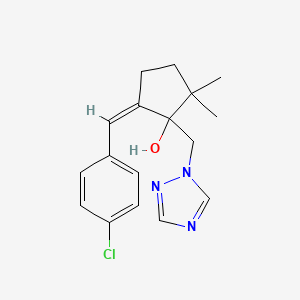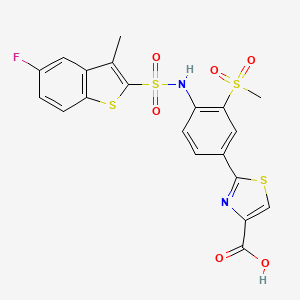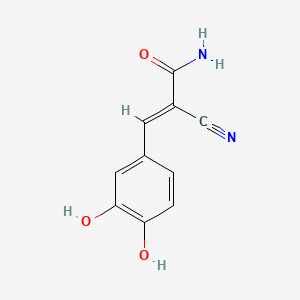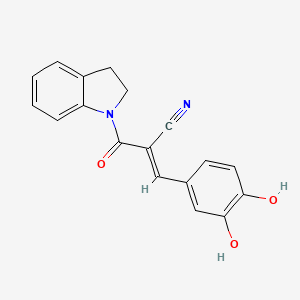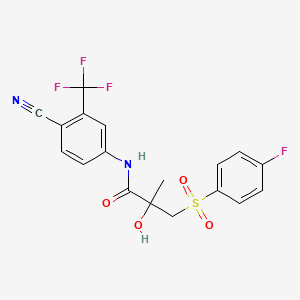
ビカルタミド
概要
説明
Bicalutamide is a nonsteroidal antiandrogen medication primarily used in the treatment of prostate cancer. It is known for its ability to block the action of androgens, which are male hormones that stimulate the growth of prostate cancer cells . Bicalutamide is often used in combination with other treatments such as gonadotropin-releasing hormone analogues or surgical removal of the testicles to manage metastatic prostate cancer .
作用機序
Target of Action
Bicalutamide is a nonsteroidal anti-androgen primarily used in the treatment of prostate cancer . The primary target of Bicalutamide is the androgen receptor (AR) . The AR is the biological target of the androgen sex hormones testosterone and dihydrotestosterone (DHT) .
Mode of Action
Bicalutamide acts as a selective antagonist of the androgen receptor . It competes with androgens for the binding of androgen receptors, consequently blocking the action of androgens of adrenal and testicular origin . This interaction inhibits the stimulation of the growth of normal and malignant prostatic tissue .
Biochemical Pathways
Bicalutamide’s action on the androgen receptor leads to a decrease in the expression of androgen-responsive genes. This results in a decrease in the production of proteins that are involved in cell proliferation, thereby inhibiting the growth of prostate cancer cells .
Pharmacokinetics
Bicalutamide is well-absorbed when taken orally . It shows extensive plasma protein binding, mainly to albumin . Bicalutamide is metabolized in the liver by hydroxylation (CYP3A4) and glucuronidation (UGT1A9) . The metabolites of Bicalutamide are excreted almost equally in urine and feces with little or no unchanged drug excreted in urine . Bicalutamide has a very long biological half-life of 6 days with a single dose and 7 to 10 days with repeated administration .
Result of Action
The molecular and cellular effects of Bicalutamide’s action include the inhibition of prostate cancer cell proliferation. By blocking the action of androgens, Bicalutamide prevents the stimulation of androgen-responsive genes, which are involved in cell proliferation . This leads to a decrease in the growth of prostate cancer cells .
Action Environment
Environmental factors can influence the action, efficacy, and stability of Bicalutamide. For instance, the presence of other drugs can affect the metabolism of Bicalutamide. Also, the Predicted Environmental Concentration (PEC) / Predicted No Effect Concentration (PNEC) is 0.01, which means that the use of Bicalutamide is predicted to present an insignificant risk to the environment .
科学的研究の応用
Bicalutamide has a wide range of scientific research applications, including:
生化学分析
Biochemical Properties
Bicalutamide acts as a pure, nonsteroidal anti-androgen with affinity for androgen receptors . It does not bind to progestogen, estrogen, or glucocorticoid receptors . Bicalutamide blocks the action of androgens of adrenal and testicular origin, which stimulate the growth of normal and malignant prostatic tissue .
Cellular Effects
Bicalutamide has significant effects on various types of cells and cellular processes. It is primarily used in the treatment of prostate cancer, where it inhibits the growth of cancer cells by blocking the action of androgens . In addition, it has been shown to reduce the allergic lesion in an animal model of allergic rhinitis .
Molecular Mechanism
Bicalutamide exerts its effects at the molecular level by binding to the androgen receptor, thereby blocking the action of androgens . This prevents androgens from stimulating the growth of normal and malignant prostatic tissue .
Temporal Effects in Laboratory Settings
Bicalutamide has a long plasma elimination half-life of one week and accumulates about 10-fold in plasma during daily administration . It is cleared almost exclusively by metabolism .
Dosage Effects in Animal Models
In animal models, the administration of Bicalutamide has been shown to significantly alleviate allergic rhinitis lesions . The study also found that there was a significant reduction in eosinophils number in Bicalutamide treated mice compared to the control group .
Metabolic Pathways
Bicalutamide is metabolized in the liver by hydroxylation and glucuronidation . The ®-bicalutamide is largely metabolized by cytochrome P450 (CYP), while glucuronidation is the predominant metabolic route for (S)-bicalutamide .
Transport and Distribution
Bicalutamide is well-absorbed when taken orally . It shows extensive plasma protein binding, mainly to albumin . It crosses the blood–brain barrier and exerts effects in the central nervous system .
Subcellular Localization
Given its mechanism of action, it can be inferred that Bicalutamide likely localizes to the cell nucleus where it binds to androgen receptors to exert its effects .
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of Bicalutamide involves several key stepsThis oxidation is typically carried out using potassium permanganate in the presence of water or a mixture of water and a water-miscible solvent . The resulting product is Bicalutamide.
Industrial Production Methods: Industrial production of Bicalutamide follows similar synthetic routes but on a larger scale. The process is designed to be simple, convenient, safe, and cost-effective. The key steps involve the preparation of the sulfide compound, followed by its oxidation to form Bicalutamide .
化学反応の分析
Types of Reactions: Bicalutamide undergoes several types of chemical reactions, including:
Substitution: Bicalutamide can undergo substitution reactions, particularly involving the cyano and trifluoromethyl groups on the phenyl ring.
Common Reagents and Conditions:
Oxidizing Agents: Potassium permanganate, peroxy acids such as m-chloroperbenzoic acid.
Solvents: Water, methanol, methylene chloride.
Major Products Formed: The major product formed from the oxidation reaction is Bicalutamide itself, which is a sulfone compound .
類似化合物との比較
- Flutamide
- Nilutamide
- Enzalutamide
- Apalutamide
- Cyproterone Acetate
- Spironolactone
Comparison:
- First-Generation Nonsteroidal Antiandrogens: Compared to flutamide and nilutamide, Bicalutamide has improved potency, efficacy, tolerability, and safety . It has largely replaced these medications in clinical practice.
- Second-Generation Nonsteroidal Antiandrogens: Compared to enzalutamide and apalutamide, Bicalutamide has inferior potency and efficacy but similar tolerability and safety .
- Steroidal Antiandrogens: Compared to cyproterone acetate and spironolactone, Bicalutamide has better selectivity in its action, superior efficacy as an antagonist of the androgen receptor, and better tolerability .
Bicalutamide’s unique properties, such as its selectivity and safety profile, make it a preferred choice in the treatment of prostate cancer .
特性
IUPAC Name |
N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-hydroxy-2-methylpropanamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LKJPYSCBVHEWIU-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(CS(=O)(=O)C1=CC=C(C=C1)F)(C(=O)NC2=CC(=C(C=C2)C#N)C(F)(F)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H14F4N2O4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2022678 | |
| Record name | Bicalutamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022678 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
430.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Bicalutamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015260 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble in chloroform and absolute ethanol; sparingly soluble in methanol; soluble in acetone and tetrahydrofuan, Practically insoluble in water at 37 °C (5 mg/1000 mL), 9.28e-03 g/L | |
| Record name | Bicalutamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01128 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | BICALUTAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7655 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Bicalutamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015260 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Bicalutamide competes with androgen for the binding of androgen receptors, consequently blocking the action of androgens of adrenal and testicular origin which stimulate the growth of normal and malignant prostatic tissue., Bicalutamide is a nonsteroidal antiandrogen that is structurally and pharmacologically related to flutamide and nilutamide. Bicalutamide inhibits the action of androgens by competitively blocking nuclear androgen receptors in target tissues such as the prostate, seminal vesicles, and adrenal cortex; blockade of androgen receptors in the hormone-sensitive tumor cells may result in growth arrest or transient tumor regression through inhibition of androgen-dependent DNA and protein synthesis. Bicalutamide is a selective antiandrogen with no androgenic or progestational activity in various animal models. The relative binding affinity of bicalutamide at the androgen receptor is more than that of nilutamide and approximately 4 times that of hydroxyflutamide, the active metabolite of flutamide., Common pharmacologic therapies for prostate cancer (ie, gonadotropin-releasing hormone [GnRH] analogs, nonsteroidal antiandrogens) when used as monotherapy initially result in increased serum testosterone concentrations, which may limit the effects of the drugs. Androgen receptors in the hypothalamus are blocked by bicalutamide, which disrupts the inhibitory feedback of testosterone on luteinizing hormone (LH) release, resulting in a temporary increase in secretion of LH; the increase in LH stimulates an increase in the production of testosterone. As GnRH analogs have potent GnRH agonist properties, testicular steroidogenesis continues during the first few weeks after initiating therapy. However, the combination of orchiectomy or GnRH analog therapy to suppress testicular androgen production and an antiandrogen to block response of remaining adrenal androgens provides maximal androgen blockade. Concomitant administration of antiandrogens such as bicalutamide in patients initiating therapy with a GnRH analog can inhibit initial androgenic stimulation and potential exacerbation of symptoms (e.g., bone pain, urinary obstruction, liver pain, impending spinal cord compression) that may occur during the first month of GnRH analog therapy., Bicalutamide was developed from a series of nonsteroidal compounds related to flutamide that showed a range of pharmacologic activity from full androgen agonist to pure antiandrogen, including progestational and antiprogestational properties. Bicalutamide is a pure antiandrogen that binds to rat, dog, and human prostate; the affinity compared with the natural ligand 5 alpha-dihydrotestosterone is low, but bicalutamide has an affinity for the rat androgen receptor approximately four times higher than hydroxyflutamide, the active metabolite of flutamide. Bicalutamide also binds to androgen receptors found in the LNCaP human prostate tumor and the Shionogi S115 mouse mammary tumor cell line, as well as androgen receptors transfected into CV-1 and HeLa cells. In all cases, bicalutamide behaves as a pure antiandrogen and inhibits gene expression and cell growth stimulated by androgen. Studies with the LNCaP cell line are particularly interesting, as these cells contain a mutated androgen receptor (codon 868, Thr-->Ala), which behaves idiosyncratically with other antiandrogens (cyproterone acetate and flutamide): both these antiandrogens act as agonists in this cell line and stimulate proliferation. Studies in vivo show that bicalutamide is a potent antiandrogen in the rat. In immature, castrated male rats treated daily with testosterone propionate, bicalutamide produces a profound inhibition of accessory sex organ (ventral prostate and seminal vesicles) growth at oral doses as low as 0.25 mg/kg; it is more active in this test than flutamide or cyproterone acetate. In mature male rats, daily oral doses of bicalutamide produce a dose-related reduction in weights of the ventral prostate glands and seminal vesicles: in this test, bicalutamide is around five times as potent as flutamide. In contrast to flutamide, which produces dose-related, marked increases in serum luteinizing hormone (LH) and testosterone as a consequence of the central inhibition of the negative feedback effects of androgens on the hypothalamic-pituitary-tests axis, bicalutamide has little effect on serum LH and testosterone; i.e., it is peripherally selective. The peripheral selectivity of bicalutamide in the rat is not due to differences between the prostate versus hypothalamic or pituitary receptors, as bicalutamide reverses the suppressive effect of testosterone on luteinizing hormone-releasing hormone (LHRH) secretion from hypothalamic slices in vitro and is as effective as flutamide at sensitizing the pituitary gland to secrete LH in response to administered LHRH. The peripheral selectivity of bicalutamide has now been shown to be due to poor penetration across the blood-brain barrier: tissue distribution studies with [3H]bicalutamide show that although it is concentrated in the organs of metabolism and secretion as well as in the prostate, the pituitary glands, and the seminal vesicles, levels in the hypothalamus and the central nervous system (CNS) are much lower than in blood. Indeed, it is probable that levels found in the CNS reflect levels of blood contamination. In dogs, bicalutamide has exquisite potency and causes dose-related atrophy of the prostate gland and epididymides; with an oral ED50 of 0.1 mg/kg, it is around 50 times as potent as flutamide in this species and also more potent than the steroidal antiandrogen WIN49596 and the 5 alpha-reductase inhibitor MK-906. Even at substantial multiples of the active dose (up to 100 mg/kg orally), bicalutamide failed to increase serum testosterone, so it is also peripherally selective in the dog., Although widely accepted as an androgen receptor antagonist, the mechanism by which it induces apoptosis remains unclear. Defining exact pathways by which bicalutamide induces its apoptotic effects would help to advance its clinical applications. /Investigators/ aimed to examine the apoptotic effects of bicalutamide at 24 hr and comment on the role of the caspases and calpains in mediating bicalutamide-induced apoptosis in androgen-dependent and androgen-independent cells. PWR-1E, PC-3 and DU-145 cells were treated with bicalutamide and assessed for apoptosis by flow cytometry at 24 hr. DU-145 cells were used to compare differences between two different metastatic receptor-negative cells and to verify apoptotic induction at 48 hr. To delineate a specific pathway of action for bicalutamide, PC-3 and PWR-1E cells were pretreated with specific inhibitors of caspase-dependent (zVAD-FMK) and caspase-independent pathways (calpain 2 inhibitor). Bicalutamide induced apoptosis in androgen-dependent PWR-1E cells via a caspase-dependent and calpain-independent mechanism. In androgen-independent PC-3 cells, bicalutamide also induced apoptosis by mechanisms that were partially inhibited by pan-caspase inhibition but were partially calpain dependent. Understanding into how bicalutamide exerts its effects in androgen-independent cells will yield further insights into the treatment of hormone-refractory disease. | |
| Record name | Bicalutamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01128 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | BICALUTAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7655 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from 1:1 (v/v) mix of ethyl acetate and petroleum ether, Fine white to off-white powder | |
CAS No. |
90357-06-5 | |
| Record name | Bicalutamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=90357-06-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bicalutamide [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0090357065 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bicalutamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01128 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | bicalutamide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759816 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Bicalutamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022678 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.126.100 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BICALUTAMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A0Z3NAU9DP | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | BICALUTAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7655 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Bicalutamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015260 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
191-193 °C, 191 - 193 °C | |
| Record name | Bicalutamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01128 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | BICALUTAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7655 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Bicalutamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015260 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details







Synthesis routes and methods IV
Procedure details









Synthesis routes and methods V
Procedure details









Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Bicalutamide?
A1: Bicalutamide acts as a competitive inhibitor of the androgen receptor (AR). [, , , , ] It binds to the ligand-binding domain of the AR, preventing androgens like testosterone and dihydrotestosterone from binding and activating the receptor. [, , , , , ]
Q2: How does Bicalutamide's binding to AR affect prostate cancer cells?
A2: By blocking AR activation, Bicalutamide inhibits the expression of androgen-regulated genes crucial for prostate cancer cell growth and survival. [, , , , , ] This leads to growth inhibition, induction of apoptosis, and reduced production of prostate-specific antigen (PSA). [, , , , , , , , , ]
Q3: Does Bicalutamide affect other signaling pathways besides the AR pathway?
A3: Yes, research suggests Bicalutamide can influence other signaling pathways, sometimes indirectly through its effects on AR. For example, it has been shown to impact the PI3K/Akt/mTOR pathway, which is involved in cell growth and proliferation. [, , ] Bicalutamide treatment has also been associated with increased levels of phospho-Akt, EGFR, and Her2, along with a reduction in PTEN levels. []
Q4: Can Bicalutamide's effect on AR influence the tumor microenvironment?
A4: Yes, Bicalutamide treatment can induce hypoxia in prostate tumors. [, , , ] This is thought to occur due to the drug's initial impact on tumor vasculature. [, , , ]
Q5: Does this Bicalutamide-induced hypoxia have consequences for tumor progression?
A5: Yes, the hypoxic environment created by Bicalutamide treatment can contribute to prostate cancer progression. [, , , ] This hypoxia can upregulate hypoxia-inducible factor 1 (HIF-1) and NF-kB, which are transcription factors involved in angiogenesis, survival, and metabolic adaptation in tumor cells. [, ] It can also lead to the selection of more aggressive cancer cells. [, , ]
Q6: Can Bicalutamide's impact on the tumor microenvironment be targeted therapeutically?
A6: Research suggests that targeting the hypoxic environment created by Bicalutamide might improve treatment outcomes. For example, combining Bicalutamide with hypoxia-activated prodrugs, like OCT1002, has shown promise in preclinical studies. [, ] These prodrugs are activated under hypoxic conditions and can enhance the antitumor effects of Bicalutamide. [, ]
Q7: What is the molecular formula and weight of Bicalutamide?
A7: The molecular formula of Bicalutamide is C18H14F4N2O4S, and its molecular weight is 430.37 g/mol.
Q8: Is there any spectroscopic data available for Bicalutamide?
A8: Spectroscopic data for Bicalutamide can be found in various resources, including pharmaceutical reference standards and research articles. Look for information on infrared (IR), ultraviolet-visible (UV-Vis), and nuclear magnetic resonance (NMR) spectroscopy.
Q9: Is Bicalutamide effective as monotherapy in prostate cancer treatment?
A10: Bicalutamide monotherapy at a dosage of 150 mg/day has demonstrated similar survival rates compared to castration in patients with advanced, non-metastatic prostate cancer. [, , , ]
Q10: What are the potential benefits of Bicalutamide monotherapy compared to castration?
A11: Studies suggest that Bicalutamide monotherapy might offer quality-of-life advantages over castration, such as better preservation of sexual interest and physical capacity. [, , , ] Additionally, preliminary data indicate that Bicalutamide monotherapy might be associated with a lower risk of osteoporosis compared to castration. [, ]
Q11: What is the role of Bicalutamide in treating locally advanced prostate cancer?
A12: Bicalutamide 150 mg/day, used as an adjuvant therapy to radical prostatectomy or radiotherapy, significantly improves progression-free survival in patients with locally advanced prostate cancer. [, , , , ] This highlights its potential role in delaying disease progression and improving outcomes in this patient population. [, , , , ]
Q12: How effective is Bicalutamide in patients with metastatic prostate cancer?
A13: While Bicalutamide can provide some benefits in metastatic prostate cancer, castration appears to offer a greater survival advantage in this setting. [] Further research is necessary to determine the optimal use of Bicalutamide in the context of metastatic disease.
Q13: Why do some patients develop resistance to Bicalutamide treatment?
A13: Resistance to Bicalutamide can occur through several mechanisms, including:
- AR mutations: Mutations in the AR can reduce Bicalutamide's binding affinity, rendering the drug less effective. [, , , , ]
- AR splice variants: Tumor cells can produce AR variants, like AR-V7, that lack the ligand-binding domain, making them insensitive to Bicalutamide. [, , ]
- AR overexpression: Increased AR expression can overwhelm Bicalutamide's inhibitory effects. [, , ]
- Activation of alternative pathways: Tumor cells can bypass AR signaling by activating other pathways, such as the PI3K/Akt/mTOR pathway, to sustain growth and survival despite Bicalutamide treatment. [, , , ]
Q14: Are there any strategies to overcome or prevent Bicalutamide resistance?
A14: Researchers are actively investigating ways to combat Bicalutamide resistance. Some potential strategies include:
- Combination therapies: Combining Bicalutamide with other agents, such as novel antiandrogens, androgen synthesis inhibitors, or drugs targeting alternative pathways, might enhance efficacy and delay resistance. [, , , ]
- Targeting AR splice variants: Developing drugs that specifically target AR splice variants, like AR-V7, may offer a way to overcome resistance mediated by these variants. []
- Targeting the tumor microenvironment: As mentioned earlier, using agents like OCT1002 to target the hypoxic environment induced by Bicalutamide may improve treatment outcomes. [, ]
Q15: How is Bicalutamide administered, and what is its typical dosage?
A17: Bicalutamide is administered orally, typically once a day. [, , ] The dosage can vary depending on the specific clinical situation, but common dosages include 50 mg/day for combination therapy and 150 mg/day for monotherapy. [, , , , , , ]
Q16: What is the half-life of Bicalutamide?
A18: Bicalutamide has a long elimination half-life, which allows for once-daily dosing. [, ] The reported half-life is approximately 6 days. [, ]
Q17: How is Bicalutamide metabolized in the body?
A19: Bicalutamide is primarily metabolized in the liver via glucuronidation. [, , ] This process involves the addition of glucuronic acid to the drug molecule, making it more water-soluble and facilitating its elimination from the body. [, , ]
Q18: What is the primary route of Bicalutamide elimination?
A20: Bicalutamide and its metabolites are primarily excreted in the feces. [, , ]
Q19: What are the most common side effects associated with Bicalutamide?
A21: The most frequently reported side effects of Bicalutamide are breast pain and gynecomastia (breast enlargement). [, , , , , ] These side effects are related to Bicalutamide's antiandrogenic activity. [, , , , , ]
Q20: Are there any serious adverse events associated with Bicalutamide?
A22: While generally well-tolerated, Bicalutamide has been associated with rare cases of hepatotoxicity (liver damage). [, ] It is essential to monitor liver function in patients receiving Bicalutamide, especially during the initial stages of treatment. [, ]
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。