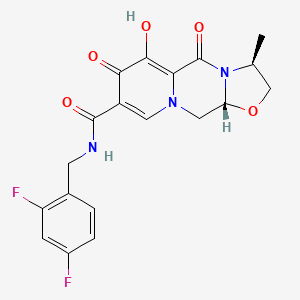
カボテグラビル
説明
カボテグラビルは、HIV/エイズの治療と予防に使用される抗レトロウイルス薬です。 インテグラーゼ鎖転移阻害剤 (INSTI) であり、ウイルスゲノムが宿主 DNA に組み込まれるのを阻止することで、ウイルスの複製を抑制します 。 カボテグラビルは、経口剤と長効性注射剤の両方で入手可能であり、HIV 治療と曝露前予防 (PrEP) に対する汎用性の高い選択肢となっています .
製法
合成経路と反応条件
カボテグラビルは、市販されている出発物質から始まり、複数の段階で合成されます。 反応条件には、一般的に水素化ナトリウムなどの強塩基と、さまざまな有機溶媒の使用が含まれます .
工業的生産方法
カボテグラビルの工業的生産は、同様の合成経路に従いますが、より大規模に行われます。このプロセスは、収量と純度を最適化するために、厳格な品質管理対策を講じています。 最終製品は、目的の使用に応じて錠剤または注射用懸濁液に製剤化されます .
科学的研究の応用
Cabotegravir has a wide range of scientific research applications, including:
Chemistry: Used as a model compound for studying integrase inhibitors and their interactions with viral DNA.
Biology: Studied for its effects on viral replication and resistance mechanisms.
Medicine: Used in clinical trials for HIV treatment and prevention, demonstrating high efficacy and safety.
Industry: Formulated into various pharmaceutical products for commercial use.
作用機序
カボテグラビルは、HIV インテグラーゼ酵素の活性部位に結合することで、その効果を発揮します。 これにより、ウイルスゲノムが宿主 DNA に転移することが阻止され、ウイルスの複製が抑制されます 。 分子標的は、インテグラーゼ酵素とウイルス DNA であり、関与する経路はウイルスの複製サイクルに関連しています .
生化学分析
Biochemical Properties
Cabotegravir interacts with the HIV-1 integrase, an enzyme crucial for the replication of the virus . By binding to the active site of this enzyme, cabotegravir prevents the integration of the viral genome into the host genome, thereby inhibiting viral replication .
Cellular Effects
Cabotegravir has a significant impact on cells infected with HIV-1. By inhibiting the action of the HIV-1 integrase, cabotegravir prevents the virus from integrating its genetic material into the host cell’s DNA, effectively stopping the replication of the virus . This results in a decrease in viral load and an increase in the number of healthy immune cells.
Molecular Mechanism
Cabotegravir exerts its effects at the molecular level by binding to the active site of the HIV-1 integrase . This binding inhibits the action of the enzyme, preventing it from integrating the viral genome into the host cell’s DNA. This inhibition of the integrase enzyme is the primary mechanism by which cabotegravir prevents the replication of the HIV-1 virus .
Temporal Effects in Laboratory Settings
In laboratory settings, cabotegravir has shown sustained antiviral activity. The long-acting injectable formulation of cabotegravir has an elimination half-life of approximately 5.6–11.5 weeks, allowing for infrequent dosing . This long duration of action contributes to the drug’s effectiveness in maintaining viral suppression over time .
Dosage Effects in Animal Models
While specific studies on cabotegravir’s dosage effects in animal models are limited, a dose-optimization study of cabotegravir in a mouse pregnancy model aimed to determine the dose that would yield plasma drug concentrations similar to those observed in humans .
Metabolic Pathways
Cabotegravir is primarily metabolized by uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) . This metabolic pathway involves the addition of a glucuronic acid group to cabotegravir, making it more water-soluble and thus easier for the body to eliminate .
Transport and Distribution
Following intramuscular administration, cabotegravir is readily absorbed and distributed within the body . It circulates mainly in the plasma, with limited partitioning in blood cells . Regarding tissue distribution, cabotegravir is found mainly in the lung, liver, renal and adrenal medulla, and skin, with limited penetration in the brain .
準備方法
Synthetic Routes and Reaction Conditions
The synthesis of cabotegravir involves multiple steps, starting from commercially available starting materialsThe reaction conditions typically involve the use of strong bases, such as sodium hydride, and various organic solvents .
Industrial Production Methods
Industrial production of cabotegravir follows similar synthetic routes but on a larger scale. The process is optimized for yield and purity, with stringent quality control measures in place. The final product is formulated into tablets or injectable suspensions, depending on the intended use .
化学反応の分析
反応の種類
カボテグラビルは、次のようなさまざまな種類の化学反応を起こします。
酸化: カボテグラビルは酸化されて、さまざまな代謝物を生成する可能性があります。
還元: 還元反応は、カボテグラビル分子の官能基を修飾することができます。
一般的な試薬と条件
これらの反応で使用される一般的な試薬には、過酸化水素などの酸化剤、水素化ホウ素ナトリウムなどの還元剤、さまざまな有機溶媒などがあります。 反応条件は、通常、目的の製品が高度な純度で得られるように制御されます .
生成される主な生成物
これらの反応から生成される主な生成物には、さまざまな代謝物やカボテグラビルの誘導体があり、それらは異なる薬理学的特性を持つ可能性があります .
科学研究への応用
カボテグラビルは、次のような幅広い科学研究に適用されています。
化学: インテグラーゼ阻害剤とそのウイルス DNA との相互作用を研究するためのモデル化合物として使用されています。
生物学: ウイルスの複製と耐性機構に対するその効果が研究されています。
類似化合物との比較
類似化合物
ドルテグラビル: 作用機序が類似している別のインテグラーゼ阻害剤。
ラルテグラビル: HIV 治療薬として承認された最初のインテグラーゼ阻害剤。
エルビテグラビル: 他の抗レトロウイルス薬と組み合わせて使用されるインテグラーゼ阻害剤.
カボテグラビルの独自性
カボテグラビルは、長効性注射製剤であるため、持続的な薬物レベルを提供し、毎日の投与の必要性を減らすことができます。 これは、毎日の投薬レジメンの遵守が難しい人にとって特に役立ちます .
特性
IUPAC Name |
(3R,6S)-N-[(2,4-difluorophenyl)methyl]-10-hydroxy-6-methyl-8,11-dioxo-4-oxa-1,7-diazatricyclo[7.4.0.03,7]trideca-9,12-diene-12-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H17F2N3O5/c1-9-8-29-14-7-23-6-12(16(25)17(26)15(23)19(28)24(9)14)18(27)22-5-10-2-3-11(20)4-13(10)21/h2-4,6,9,14,26H,5,7-8H2,1H3,(H,22,27)/t9-,14+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WCWSTNLSLKSJPK-LKFCYVNXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1COC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1CO[C@H]2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H17F2N3O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID50146982 | |
| Record name | GSK-1265744 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50146982 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
405.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Cabotegravir binds to the active site of HIV integrase, preventing strand transfer of the viral genome into the host genome, and preventing replication of the virus. | |
| Record name | Cabotegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11751 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1051375-10-0 | |
| Record name | Cabotegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1051375-10-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cabotegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1051375100 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cabotegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11751 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | GSK-1265744 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50146982 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (3S, 11AR)-N-[(2,4-DIFLUOROPHENYL) METHYL] - 6- HYDROXY-3-METHYL-5,7-DIOXO-2,3,5,7,11,11A- HEXADROOXAZOLO[3,2-A] PYRIDO[1,2-D]PYRAZINE-8-CARBOXAMIDE | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CABOTEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HMH0132Z1Q | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Q1: What is the mechanism of action of cabotegravir?
A1: Cabotegravir is a potent integrase strand transfer inhibitor (INSTI) that acts by binding to the integrase enzyme of HIV-1. This binding prevents the integration of viral DNA into the host cell's genome, thus blocking viral replication. [, , , ]
Q2: How does the inhibition of HIV-1 integrase by cabotegravir affect the virus lifecycle?
A2: By inhibiting integrase, cabotegravir prevents the formation of proviral DNA, which is essential for the production of new viral proteins and the establishment of a persistent infection. [, , ]
Q3: Does cabotegravir demonstrate activity against HIV-2?
A3: Yes, in vitro studies have demonstrated that cabotegravir exhibits antiviral activity against HIV-2 isolates, with EC50 values in the low to subnanomolar range, comparable to its activity against HIV-1. []
Q4: What is the molecular formula and weight of cabotegravir?
A4: Cabotegravir has the molecular formula C28H27F2N7O4 and a molecular weight of 555.56 g/mol. []
Q5: How do structural modifications of cabotegravir influence its activity and solubility?
A5: While the exact SAR of cabotegravir's solubility is not fully elucidated in these papers, research suggests that specific structural features contribute to its low aqueous solubility, which is crucial for its long-acting formulation. [] One study investigating resistance mechanisms identified that specific mutations in the HIV integrase enzyme, like L74I/Q148R, can reduce cabotegravir's susceptibility. []
Q6: How is cabotegravir formulated for long-acting administration?
A6: Cabotegravir is formulated as a nanosuspension for long-acting intramuscular (IM) injection. This formulation allows for slow drug release from the muscle, resulting in sustained therapeutic concentrations for extended periods. [, , ]
Q7: How does the stability of cabotegravir in its injectable formulation compare to other formulations?
A7: The long-acting injectable formulation of cabotegravir exhibits a significantly longer half-life compared to the oral formulation, allowing for less frequent dosing intervals (monthly or even bimonthly). [, ] This difference in half-life is attributed to the slow release of the drug from the intramuscular depot.
Q8: How is cabotegravir absorbed, distributed, metabolized, and excreted (ADME)?
A8: Cabotegravir is primarily metabolized by UDP-glucuronosyltransferase (UGT) 1A1 and UGT1A9 enzymes in the liver. [] Following oral administration, approximately 27% of the dose is recovered in the urine, primarily as the glucuronide metabolite (M1). [] Cabotegravir demonstrates minimal intestinal and renal metabolism. []
Q9: What is the impact of renal impairment on cabotegravir exposure?
A9: Severe renal impairment (creatinine clearance <30 mL/min) does not significantly impact plasma cabotegravir exposure. Therefore, dose adjustment is not required in patients with renal impairment who are not on dialysis. []
Q10: How does a high-fat meal affect the pharmacokinetics of cabotegravir?
A10: A high-fat meal moderately increases cabotegravir exposure (area under the curve and maximum concentration) by approximately 14%, which is not considered clinically significant. []
Q11: How does obesity impact the exposure of long-acting cabotegravir and rilpivirine?
A12: Studies using physiologically based pharmacokinetic modeling suggest that obesity can reduce the exposure of both drugs, particularly in individuals with a BMI > 35 kg/m2. This reduction might lead to suboptimal drug concentrations, especially with less frequent dosing schedules. []
Q12: Does age affect the pharmacokinetics of long-acting cabotegravir and rilpivirine?
A13: PBPK modeling studies suggest that older individuals may have higher exposure to LA cabotegravir/rilpivirine compared to younger individuals. This difference highlights the need to consider age-related pharmacokinetic variations in this population. []
Q13: What is the impact of oral lead-in on long-acting cabotegravir pharmacokinetics?
A14: Studies have shown that the use of an oral lead-in before initiating long-acting cabotegravir injections does not significantly affect the drug's overall exposure. This finding supports the optional use of oral lead-in. []
Q14: What preclinical studies have been conducted to evaluate the efficacy of long-acting cabotegravir?
A15: Preclinical studies in macaque models demonstrated that long-acting cabotegravir provided high protection against both rectal and vaginal HIV transmission at clinically achievable drug concentrations. These findings supported the drug's development for pre-exposure prophylaxis (PrEP). [, ]
Q15: What are the key findings from clinical trials evaluating long-acting cabotegravir for HIV treatment and prevention?
A16:* HPTN 083 & HPTN 084: Demonstrated superior efficacy of long-acting cabotegravir compared to daily oral tenofovir disoproxil fumarate–emtricitabine (TDF–FTC) for PrEP in men who have sex with men (MSM), transgender women, and cisgender women in sub-Saharan Africa. [, ] * ECLAIR: Phase IIa study showcasing the safety, tolerability, and acceptability of cabotegravir LA for PrEP in healthy men. []* LATTE: Phase IIb study demonstrating the efficacy of daily oral cabotegravir + rilpivirine compared to efavirenz + 2 NRTIs in treatment-naïve adults with HIV-1. []* LATTE-2: Phase IIb study showing comparable efficacy of long-acting cabotegravir + rilpivirine (dosed every 4 or 8 weeks) to daily oral ART in treatment-naïve adults with HIV-1. [] * FLAIR: Phase III study demonstrating non-inferiority of monthly long-acting cabotegravir + rilpivirine versus daily oral dolutegravir/abacavir/lamivudine for maintaining virologic suppression in treatment-naïve individuals. []* ATLAS: Phase III study confirming the efficacy of monthly cabotegravir + rilpivirine injections in maintaining HIV suppression in treatment-experienced patients. []* ATLAS-2M: Supported the efficacy of cabotegravir + rilpivirine injections administered every 8 weeks. []
Q16: What are the known resistance mechanisms to cabotegravir?
A17: Resistance to cabotegravir can emerge through mutations in the HIV-1 integrase gene. While the L74I polymorphism alone doesn't significantly impact cabotegravir susceptibility, the combination of L74I with Q148R mutations can lead to reduced susceptibility. [] Additionally, mutations like E138A/G140A/G163R/Q148R or E138K/G140A/S147G/Q148K have been shown to confer high-level resistance to both cabotegravir and bictegravir, highlighting potential cross-resistance within the INSTI class. []
Q17: Does the HIV-1 subtype influence the risk of treatment failure with cabotegravir + rilpivirine?
A18: Yes, clinical trials have shown that individuals infected with HIV-1 subtypes A6/A1, initially classified as A1, have an increased risk of virologic failure when treated with cabotegravir + rilpivirine. This finding emphasizes the importance of considering HIV-1 subtype when prescribing this treatment. [, ]
Q18: What are the most common side effects associated with long-acting cabotegravir injections?
A19: The most commonly reported side effect is injection site reactions (ISRs), including pain, induration, and swelling. These reactions are typically mild to moderate in severity and tend to decrease in frequency with subsequent injections. [, ]
Q19: What analytical techniques are used to quantify cabotegravir in biological samples?
A20: Several analytical methods, including high-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS) and tandem mass spectrometry (MS/MS), have been developed and validated for the quantification of cabotegravir in biological samples. [, , ] These methods demonstrate high sensitivity, accuracy, and reproducibility for therapeutic drug monitoring.
Q20: Why is the low solubility of cabotegravir important for its long-acting formulation?
A21: The low solubility of cabotegravir in aqueous solutions is a key factor contributing to its slow and sustained release from the intramuscular injection site, allowing for a longer duration of action and less frequent dosing. [, ]
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。