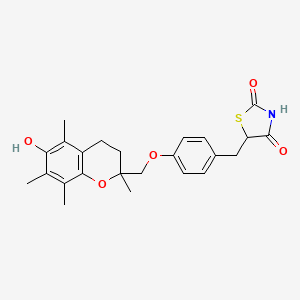
曲格列酮
概述
描述
曲格列酮是一种抗糖尿病和抗炎药物,属于噻唑烷二酮类。它最初开发用于治疗2型糖尿病,通过改善胰岛素敏感性。曲格列酮于1983年获得专利,并于1997年获准用于医疗用途。 但由于与严重肝毒性有关,该药物于2000年被撤出市场 .
科学研究应用
曲格列酮因其在各个领域的应用而被广泛研究:
化学: 用作模型化合物,研究噻唑烷二酮化学及其与其他分子的相互作用。
生物学: 研究其对细胞过程的影响,包括细胞凋亡和细胞周期调控。
作用机制
曲格列酮通过激活过氧化物酶体增殖物激活受体 (PPAR),特别是 PPARγ 以及在较小程度上激活 PPARα,来发挥其作用。这些核受体调节参与葡萄糖和脂质代谢的基因转录。曲格列酮降低肝脏葡萄糖输出,增加骨骼肌中胰岛素依赖性葡萄糖处置。 此外,它还通过降低核因子 kappa-B (NF-κB) 活性并增加其抑制剂 (IκB) 来发挥抗炎作用 .
类似化合物:
吡格列酮: 另一种噻唑烷二酮,具有类似的作用机制,但安全性更高。
罗格列酮: 与吡格列酮类似,它激活 PPARγ,但与心血管风险有关.
独特性: 曲格列酮是第一个用于临床的噻唑烷二酮,使其成为该药物类别中的先驱。其独特的结构包括一个色满环,而其他噻唑烷二酮中不存在这种环。 尽管被撤回,但曲格列酮为开发更安全更有效的抗糖尿病药物提供了宝贵的见解 .
生化分析
Biochemical Properties
Troglitazone works by activating peroxisome proliferator-activated receptors (PPARs). It is a ligand to both PPARα and – more strongly – PPARγ . Troglitazone also contains an α-Tocopherol moiety, potentially giving it vitamin E-like activity in addition to its PPAR activation . It has been shown to reduce inflammation . Troglitazone decreases hepatic glucose output and increases insulin-dependent glucose disposal in skeletal muscle .
Cellular Effects
Troglitazone improves insulin responsiveness in skeletal muscle of patients with type 2 diabetes by facilitating glucose transport activity, which thereby leads to increased rates of muscle glycogen synthesis and glucose oxidation . Troglitazone has been shown to induce apoptosis in various hepatic and nonhepatic cells .
Molecular Mechanism
Troglitazone’s mechanism of action is thought to involve binding to nuclear receptors (PPAR) that regulate the transcription of a number of insulin-responsive genes critical for the control of glucose and lipid metabolism . Troglitazone is a ligand to both PPARα and PPARγ, with a higher affinity for PPARγ .
Temporal Effects in Laboratory Settings
In a 6-month, randomized, double-blind, placebo-controlled study, troglitazone was found to significantly improve HbA1c and fasting serum glucose, while lowering insulin and C-peptide in patients with type 2 diabetes . No signs of hepatotoxicity were apparent at 2 weeks of treatment .
Dosage Effects in Animal Models
Prolonged administration of troglitazone can superimpose oxidant stress, potentiate mitochondrial damage, and induce delayed hepatic necrosis in mice with genetically compromised mitochondrial function .
Metabolic Pathways
Troglitazone decreases hepatic glucose output and increases insulin-dependent glucose disposal in skeletal muscle . Its mechanism of action is thought to involve binding to nuclear receptors (PPAR) that regulate the transcription of a number of insulin-responsive genes critical for the control of glucose and lipid metabolism .
Transport and Distribution
Troglitazone contains the structure of a unique chroman ring of vitamin E, and this structure has the potential to undergo metabolic biotransformation to form quinone metabolites, phenoxy radical intermediate, and epoxide species .
Subcellular Localization
Given its mechanism of action involving nuclear receptors (PPAR), it can be inferred that Troglitazone likely interacts with these receptors in the cell nucleus .
准备方法
合成路线和反应条件: 曲格列酮合成涉及几个关键步骤:
噻唑烷二酮环的形成: 这通常通过在碱性条件下使噻唑烷-2,4-二酮与适当的苄基卤化物反应来实现。
色满环的连接: 色满环是维生素E的结构类似物,通过涉及合适的色满衍生物和苄基化噻唑烷二酮中间体的亲核取代反应引入。
工业生产方法: 曲格列酮的工业生产遵循类似的合成路线,但规模更大。该过程涉及优化反应条件,以最大限度地提高产量和纯度,同时最大限度地减少副产物。 高效液相色谱 (HPLC) 等先进技术用于纯化 .
反应类型:
氧化: 曲格列酮发生氧化反应,特别是在肝脏中,导致形成反应性代谢物。
还原: 还原反应不太常见,但在特定条件下会发生。
取代: 亲核取代反应参与曲格列酮的合成.
常用试剂和条件:
氧化剂: 肝脏中的细胞色素 P450 酶。
还原剂: 在受控条件下特定还原剂。
亲核试剂: 合成过程中使用的各种亲核试剂.
主要产品:
反应性代谢物: 在氧化过程中形成,导致肝毒性。
稳定代谢物: 在代谢过程中形成.
相似化合物的比较
Pioglitazone: Another thiazolidinedione with a similar mechanism of action but a better safety profile.
Rosiglitazone: Similar to pioglitazone, it activates PPARγ but has been associated with cardiovascular risks.
Uniqueness: Troglitazone was the first thiazolidinedione introduced for clinical use, making it a pioneer in this drug class. Its unique structure includes a chroman ring, which is not present in other thiazolidinediones. Despite its withdrawal, troglitazone provided valuable insights into the development of safer and more effective antidiabetic drugs .
属性
IUPAC Name |
5-[[4-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydrochromen-2-yl)methoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C24H27NO5S/c1-13-14(2)21-18(15(3)20(13)26)9-10-24(4,30-21)12-29-17-7-5-16(6-8-17)11-19-22(27)25-23(28)31-19/h5-8,19,26H,9-12H2,1-4H3,(H,25,27,28) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GXPHKUHSUJUWKP-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C2=C(CCC(O2)(C)COC3=CC=C(C=C3)CC4C(=O)NC(=O)S4)C(=C1O)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C24H27NO5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8023719 | |
| Record name | Troglitazone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
441.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Troglitazone is a thiazolidinedione antidiabetic agent that lowers blood glucose by improving target cell response to insulin. It has a unique mechanism of action that is dependent on the presence of insulin for activity. Troglitazone decreases hepatic glucose output and increases insulin dependent glucose disposal in skeletal muscle. Its mechanism of action is thought to involve binding to nuclear receptors (PPAR) that regulate the transcription of a number of insulin responsive genes critical for the control of glucose and lipid metabolism. Troglitazone is a ligand to both PPARα and PPARγ, with a highter affinity for PPARγ. The drug also contains an α-tocopheroyl moiety, potentially giving it vitamin E-like activity. Troglitazone has been shown to reduce inflammation, and is associated with a decrase in nuclear factor kappa-B (NF-κB) and a concomitant increase in its inhibitor (IκB). Unlike sulfonylureas, troglitazone is not an insulin secretagogue. | |
| Record name | Troglitazone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
97322-87-7 | |
| Record name | Troglitazone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=97322-87-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Troglitazone [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0097322877 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Troglitazone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Troglitazone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Melting Point |
184-186 °C | |
| Record name | Troglitazone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details







Synthesis routes and methods III
Procedure details






Synthesis routes and methods IV
Procedure details







Synthesis routes and methods V
Procedure details









Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。
![(6aS,6bS,10R,10aR,11aS,11bR)-10,11b-dimethyl-1,2,5,6,6a,6b,7,8,10,10a,11,11a-dodecahydrobenzo[a]fluorene-3,9-dione](/img/structure/B1681509.png)
![[(2S)-1-[[1-[[(3R,4S)-1-cyclohexyl-3,4-dihydroxy-6-methylheptan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl] morpholine-4-carboxylate](/img/structure/B1681510.png)
![3-chloro-N'-(3-pyridin-4-ylpropanoyl)-6H-benzo[b][1,4]benzoxazepine-5-carbohydrazide;hydrochloride](/img/structure/B1681513.png)
![3-[(2S)-7-[3-[2-(cyclopropylmethyl)-3-methoxy-4-(methylcarbamoyl)phenoxy]propoxy]-8-propyl-3,4-dihydro-2H-chromen-2-yl]propanoic acid](/img/structure/B1681517.png)