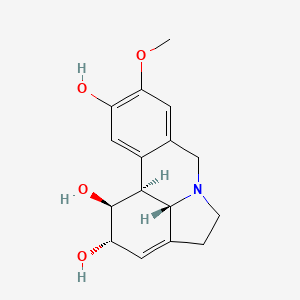
假副石蒜碱
描述
Pseudolycorine is an alkaloid isolated from the plant Narcissus tazetta L. var. Chinensis Roem, belonging to the Amaryllidaceae family . This compound has garnered interest due to its diverse biological activities, including antitumor, antiviral, and immunosuppressive properties .
科学研究应用
作用机制
Target of Action
Pseudolycorine primarily targets myeloid-derived suppressor cells (MDSCs) and T cells . MDSCs are a heterogeneous population of immune cells from the myeloid lineage that have potent immune-suppressing activities . T cells are a type of white blood cell that play a central role in cell-mediated immunity .
Mode of Action
Pseudolycorine inhibits the proliferation of activated T cells by blocking the cell cycle in the G0/G1 phase . In the case of MDSCs, pseudolycorine significantly inhibits their proliferation, expansion, and differentiation into monocyte-like MDSCs (M-MDSCs) in a dose-dependent manner .
Biochemical Pathways
Pseudolycorine significantly inhibits the expression of IL-6, IL-17, and IFN-γ . These cytokines are involved in the inflammatory response and the regulation of immune responses. The inhibition of these cytokines can lead to a decrease in inflammation and immune response .
Pharmacokinetics
It is known that pseudolycorine can be administered in vivo, as evidenced by studies where mice were treated with pseudolycorine . More research is needed to fully understand the ADME properties of pseudolycorine.
Result of Action
Pseudolycorine’s action results in the alleviation of experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis . This is achieved through the inhibition of inflammatory infiltration, demyelination, and the infiltration of MDSCs and Th17 cells into the spinal cord .
Action Environment
The action of pseudolycorine can be influenced by various environmental factors. For instance, the extraction of pseudolycorine from the bulb of Lycoris radiata can be affected by the extraction method used . .
生化分析
Biochemical Properties
Pseudolycorine plays a crucial role in various biochemical reactions. It has been shown to interact with several enzymes, proteins, and other biomolecules. For instance, pseudolycorine can inhibit protein synthesis by stabilizing polysomes in HeLa cells . Additionally, it exhibits cytotoxic effects against cancer cell lines by interfering with cellular processes such as DNA replication and repair . Pseudolycorine also interacts with enzymes involved in metabolic pathways, further influencing cellular functions.
Cellular Effects
Pseudolycorine exerts significant effects on various types of cells and cellular processes. In cancer cells, pseudolycorine induces cytotoxicity by inhibiting cell proliferation and promoting apoptosis . It affects cell signaling pathways, including those involved in cell cycle regulation and apoptosis. Pseudolycorine has also been shown to influence gene expression, leading to changes in the expression of genes related to cell growth and survival . Furthermore, pseudolycorine impacts cellular metabolism by altering the activity of key metabolic enzymes.
Molecular Mechanism
The molecular mechanism of pseudolycorine involves several key interactions at the molecular level. Pseudolycorine binds to specific biomolecules, including proteins and nucleic acids, thereby modulating their activity. It inhibits the activity of certain enzymes, such as those involved in protein synthesis and DNA replication . Additionally, pseudolycorine can activate or inhibit signaling pathways, leading to changes in gene expression and cellular responses. These molecular interactions contribute to the compound’s diverse biological effects.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of pseudolycorine have been observed to change over time. Pseudolycorine exhibits stability under specific conditions, but it can degrade over time, leading to a reduction in its biological activity . Long-term studies have shown that pseudolycorine can have sustained effects on cellular function, including prolonged inhibition of cell proliferation and induction of apoptosis . These temporal effects are important considerations for its potential therapeutic applications.
Dosage Effects in Animal Models
The effects of pseudolycorine vary with different dosages in animal models. At lower doses, pseudolycorine has been shown to exhibit therapeutic effects, such as antitumor activity and inhibition of viral replication . At higher doses, pseudolycorine can induce toxic effects, including hepatotoxicity and nephrotoxicity . These dosage-dependent effects highlight the importance of determining the optimal therapeutic dose for potential clinical applications.
Metabolic Pathways
Pseudolycorine is involved in several metabolic pathways, interacting with enzymes and cofactors that regulate its metabolism. It can influence metabolic flux and alter the levels of specific metabolites . Pseudolycorine has been shown to affect pathways related to amino acid metabolism, nucleotide synthesis, and energy production . These interactions contribute to its overall biological activity and therapeutic potential.
Transport and Distribution
Within cells and tissues, pseudolycorine is transported and distributed through specific mechanisms. It interacts with transporters and binding proteins that facilitate its uptake and distribution . Pseudolycorine can accumulate in certain cellular compartments, influencing its localization and activity. The transport and distribution of pseudolycorine are critical factors that determine its bioavailability and therapeutic efficacy.
Subcellular Localization
Pseudolycorine exhibits specific subcellular localization, which affects its activity and function. It can be directed to specific compartments or organelles through targeting signals and post-translational modifications . For example, pseudolycorine has been observed to localize in the nucleus, where it can interact with DNA and modulate gene expression . Understanding the subcellular localization of pseudolycorine is essential for elucidating its mechanism of action and potential therapeutic applications.
准备方法
Pseudolycorine can be extracted from the bulbs of Narcissus tazetta using a series of steps involving preparation of lycoris bulbiferous dry powder, wet mixing with a dilute alkali solution, and leaching with ethyl acetate . The synthetic routes for pseudolycorine involve complex organic reactions, but detailed synthetic pathways are not widely documented in the literature.
化学反应分析
Pseudolycorine undergoes various chemical reactions, including:
Oxidation: Pseudolycorine can be oxidized under specific conditions, although detailed reagents and conditions are not extensively documented.
Reduction: Reduction reactions of pseudolycorine are less common in the literature.
Substitution: Pseudolycorine can undergo substitution reactions, particularly involving its functional groups
相似化合物的比较
Pseudolycorine is structurally similar to other Amaryllidaceae alkaloids such as lycorine, dihydrolycorine, and ungeremine . . Other similar compounds include:
Lycorine: Known for its antitumor activity via the JAK/STAT pathway.
Dihydrolycorine: Exhibits similar biological activities but with different potency.
Ungeremine: Another Amaryllidaceae alkaloid with distinct pharmacological properties.
Pseudolycorine’s unique combination of biological activities and its potential for therapeutic applications make it a compound of significant interest in scientific research.
属性
IUPAC Name |
(1S,14S,15S,16S)-5-methoxy-9-azatetracyclo[7.6.1.02,7.012,16]hexadeca-2,4,6,12-tetraene-4,14,15-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H19NO4/c1-21-13-5-9-7-17-3-2-8-4-12(19)16(20)14(15(8)17)10(9)6-11(13)18/h4-6,12,14-16,18-20H,2-3,7H2,1H3/t12-,14-,15+,16+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CKAHWDNDUGDSLE-ARLBYUKCSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C2C3C(C(C=C4C3N(CC4)CC2=C1)O)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=C(C=C2[C@@H]3[C@@H]([C@H](C=C4[C@H]3N(CC4)CC2=C1)O)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H19NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID00231711 | |
| Record name | 9-Methoxy-3,12-didehydrogalanthan-1,2,10-triol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID00231711 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
289.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
29429-03-6, 82372-67-6 | |
| Record name | Pseudolycorine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=29429-03-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pseudolycorine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0029429036 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | 9-Methoxy-3,12-didehydrogalanthan-1,2,10-triol | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0082372676 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | 9-Methoxy-3,12-didehydrogalanthan-1,2,10-triol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID00231711 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
ANone: [, ] Pseudolycorine has been shown to inhibit the proliferation, expansion, and differentiation of MDSCs, particularly the monocyte-like MDSC subset (M-MDSCs). This effect is mediated, at least in part, by suppressing the response of MDSCs to interleukin-6 (IL-6) and granulocyte-macrophage colony-stimulating factor (GM-CSF), key cytokines involved in MDSC expansion and differentiation. This suppression of MDSC activity contributes to Pseudolycorine's ability to ameliorate Th17 cell-mediated central nervous system autoimmunity in experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis.
ANone: [] Studies suggest that Pseudolycorine can ameliorate the severity of Th17 cell-mediated EAE by restraining the expansion of MDSCs. This is significant because EAE is characterized by the infiltration of a large number of MDSCs, which can exacerbate the disease progression.
ANone: [, , ] Pseudolycorine has the molecular formula C16H19NO4 and a molecular weight of 289.33 g/mol.
ANone: [, ] Extensive spectroscopic data, including infrared (IR), ultraviolet (UV), nuclear magnetic resonance (NMR), and optical rotatory dispersion (ORD) spectra, has been used to elucidate the structure of Pseudolycorine. These analyses confirmed the presence of specific functional groups like hydroxyl, methoxy, and aromatic rings, and provided insights into its three-dimensional structure.
ANone: The currently available research primarily focuses on the biological activity and chemical structure of Pseudolycorine. Further research is needed to explore its material compatibility and stability under diverse conditions.
ANone: Current research primarily focuses on the biological activity of Pseudolycorine. There is no reported evidence suggesting catalytic properties for this alkaloid.
ANone: While the research provided does not explicitly detail computational studies on Pseudolycorine, such approaches could be valuable for further investigation. Computational models could help predict the binding affinity of Pseudolycorine to potential targets, explore structure-activity relationships, and guide the design of novel derivatives with enhanced properties.
ANone: Research on Pseudolycorine stability under various conditions and formulation approaches is currently limited. Investigating these aspects is crucial for developing stable and effective drug delivery systems for this alkaloid.
ANone: The provided research primarily focuses on the isolation, structural characterization, and initial biological evaluation of Pseudolycorine and related Amaryllidaceae alkaloids. Comprehensive investigations into these aspects (SHE regulations, detailed pharmacokinetics, extensive in vivo efficacy, resistance mechanisms, long-term toxicology studies, targeted drug delivery approaches, biomarker discovery, etc.) are necessary to fully understand the therapeutic potential and risks associated with Pseudolycorine.
ANone: [, , , ] The isolation of Pseudolycorine from Narcissus tazetta L. var. Chinensis Roem represents an early milestone in its research. Subsequent studies focused on developing efficient isolation methods, like the ammonium reineckate precipitation method. Structural elucidation using various spectroscopic techniques, including IR, UV, NMR, and ORD, marked significant progress in understanding this alkaloid. Research on its biological activity, particularly its antitumor and antiviral properties, has further fueled interest in Pseudolycorine.
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。