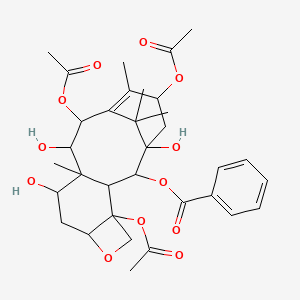
长春新碱
描述
Vincristine is a vinca alkaloid derived from the Madagascar periwinkle plant, Catharanthus roseus . It is a chemotherapy medication used to treat various types of cancer, including acute lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, neuroblastoma, and small cell lung cancer . Vincristine works by inhibiting cell division, making it a crucial component in cancer treatment regimens .
作用机制
Target of Action
Vincristine, a vinca alkaloid, primarily targets the protein tubulin, a key component of the microtubules in cells . Microtubules play a crucial role in maintaining cell structure and function, particularly during cell division .
Mode of Action
Vincristine binds to tubulin, inhibiting the polymerization of tubulin dimers and preventing the formation of microtubules . This interaction disrupts the formation of the mitotic spindle, a structure essential for chromosome separation during cell division . As a result, cells are arrested at metaphase, a stage in mitosis, leading to cell death .
Biochemical Pathways
The primary biochemical pathway affected by vincristine is the cell cycle, specifically the M and S phases . By disrupting microtubule dynamics, vincristine causes mitotic arrest, halting the cell cycle and leading to cell death . This disruption can also interfere with amino acid, cyclic AMP, and glutathione metabolism, as well as calmodulin-dependent Ca2±transport ATPase activity, and cellular respiration .
Pharmacokinetics
The pharmacokinetics of vincristine involve its absorption, distribution, metabolism, and excretion (ADME). Vincristine is administered intravenously, bypassing the absorption phase . It is metabolized primarily in the liver by the CYP3A5 enzyme . Vincristine’s neurotoxicity, a dose-limiting factor, is thought to be influenced by genetic variations in drug-metabolizing enzymes and transporters .
Result of Action
The molecular effect of vincristine’s action is the disruption of microtubule formation, leading to cell cycle arrest . On a cellular level, this results in the death of rapidly dividing cells, particularly cancer cells . This can also affect healthy cells, leading to side effects such as peripheral neuropathy .
Action Environment
Environmental factors can influence the action and efficacy of vincristine. For instance, the presence of other drugs used in combination therapies can impact vincristine’s effectiveness and toxicity profile . Additionally, genetic variations in patients can influence the drug’s metabolism and transport, potentially affecting its efficacy and toxicity .
科学研究应用
Vincristine has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry . In chemistry, it is used as a model compound for studying the mechanisms of alkaloid biosynthesis and the development of new synthetic methodologies . In biology, vincristine is used to study cell division and the effects of microtubule inhibition on cellular processes . In medicine, it is a key component of chemotherapy regimens for various cancers, helping to inhibit the growth and spread of cancer cells . In industry, vincristine is used in the development of new drug formulations and delivery systems to improve its efficacy and reduce side effects .
生化分析
Biochemical Properties
Vincristine is a vinca alkaloid that binds to the microtubular proteins of the mitotic spindle . This interaction leads to the crystallization of the microtubule and mitotic arrest or cell death . Vincristine has some immunosuppressant effect . The vinca alkaloids are considered to be cell cycle phase-specific .
Cellular Effects
Vincristine works by stopping the cancer cells from separating into 2 new cells, thereby stopping the growth of the cancer . It achieves this through a process that disrupts the formation of microtubules, which affects cell division and eventually leads to apoptosis .
Molecular Mechanism
The molecular mechanism of Vincristine involves its binding to the tubulin protein, stopping the tubulin dimers from polymerizing to form microtubules . This causes the cell to be unable to separate its chromosomes during the metaphase . The cell then undergoes apoptosis . The vincristine molecule inhibits leukocyte production and maturation .
Temporal Effects in Laboratory Settings
It is known that within 15 to 30 minutes after injection, over 90% of the drug is distributed from the blood into tissue, where it remains tightly, but not irreversibly, bound .
Dosage Effects in Animal Models
In animal models, vincristine has shown high efficiency (86.6%) for treatment of transmissible venereal tumor in canines at the dose of 0.5 mg/m^2 of body surface (IV), on 7-14 days intervals . One tumor (6.6%) was refractory to vincristine, while at the treatment beginning a mass reduction was observed .
Metabolic Pathways
Vincristine may interfere with amino acid, cyclic AMP, and glutathione metabolism . It may also interfere with calmodulin-dependent Ca^2+ -transport ATPase activity, cellular respiration, and nucleic acid and lipid biosynthesis .
Transport and Distribution
Vincristine is transported and distributed within cells and tissues primarily through the bloodstream . It is given intravenously and can be administered through a drip into the arm or hand . Over 90% of the drug is distributed from the blood into tissue within 15 to 30 minutes after injection .
Subcellular Localization
The subcellular localization of Vincristine is primarily at the mitotic spindle, where it binds to the microtubular proteins . This binding leads to the crystallization of the microtubule and mitotic arrest or cell death .
准备方法
Vincristine is primarily obtained from the natural alkaloid plant source, Catharanthus roseus . The extraction process involves isolating the compound from the plant material, followed by purification steps to obtain the active ingredient . Industrial production methods often involve the use of advanced extraction techniques and purification processes to ensure the compound’s efficacy and safety .
化学反应分析
Vincristine undergoes various chemical reactions, including oxidation, reduction, and substitution . Common reagents used in these reactions include oxidizing agents, reducing agents, and nucleophiles . The major products formed from these reactions depend on the specific conditions and reagents used . For example, oxidation of vincristine can lead to the formation of different oxidized derivatives, while reduction can yield reduced forms of the compound .
相似化合物的比较
These compounds share a similar mechanism of action, targeting microtubule formation and inhibiting cell division . vincristine is unique in its specific clinical applications and its relatively lower bone marrow suppression compared to other vinca alkaloids . Vinblastine, for example, is used to treat different types of cancer and has a different toxicity profile . Vindesine and vinflunine are also used in cancer treatment but have distinct pharmacokinetic properties and clinical uses .
属性
CAS 编号 |
57-22-7 |
|---|---|
分子式 |
C46H56N4O10 |
分子量 |
825.0 g/mol |
IUPAC 名称 |
methyl (10S,11R,12R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-8-formyl-10-hydroxy-5-methoxy-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate |
InChI |
InChI=1S/C46H56N4O10/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3/t28-,37?,38?,39-,42+,43-,44?,45+,46+/m1/s1 |
InChI 键 |
OGWKCGZFUXNPDA-DLBZMDDPSA-N |
杂质 |
3'-hydroxyvincristine; 4'-deoxyvincristine; N-desmethylvinblastine; deacetylvincristine; deacetylvinblastine; vinblastine; leurosine; formylleurosine |
SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
手性 SMILES |
CC[C@@]1(C[C@@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7[C@@](C=CC9)([C@H]([C@@](C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
规范 SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
外观 |
White to off-white, odorless amorphous or crystalline powder |
颜色/形态 |
Blades from methanol |
熔点 |
424 to 428 °F (NTP, 1992) 218-220 °C |
| 57-22-7 | |
物理描述 |
Vincristine appears as a white crystalline solid. Melting point 218 °C. Used as an antineoplastic. |
保质期 |
STERILE SOLN IN EITHER H2O OR PHYSIOLOGICAL SALINE STORED IN REFRIGERATOR FOR UP TO 2 WK WITHOUT SIGNIFICANT LOSS OF POTENCY |
溶解度 |
WHITE TO SLIGHTLY YELLOW, AMORPHOUS OR CRYSTALLINE POWDER; ODORLESS, HYGROSCOPIC; FREELY SOL IN WATER /VINCRISTINE SULFATE USP/ |
同义词 |
cellcristin Citomid Farmistin Leurocristine Oncovin Oncovine Onkocristin PFS, Vincasar Sulfate, Vincristine Vincasar Vincasar PFS Vincristin Bristol Vincristin medac Vincristine Vincristine Sulfate Vincrisul Vintec |
产品来源 |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid;hydrochloride](/img/structure/B1662843.png)
![(6S)-1-[[4-(dimethylamino)-3-methylphenyl]methyl]-5-(2,2-diphenylacetyl)-6,7-dihydro-4H-imidazo[4,5-c]pyridine-6-carboxylic acid;2,2,2-trifluoroacetic acid](/img/structure/B1662854.png)