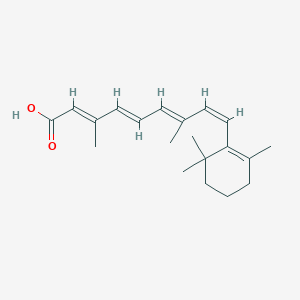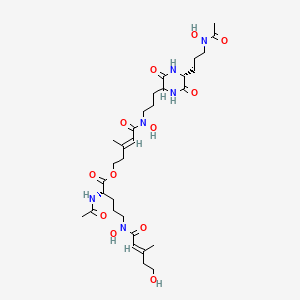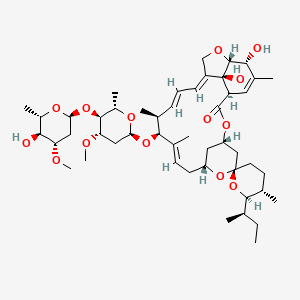
伊维菌素 B1a
描述
Ivermectin B1a is a broad-spectrum antiparasitic agent belonging to the avermectin family of medications. It was discovered in 1975 and initially used in veterinary medicine to prevent and treat heartworm and acariasis. Approved for human use in 1987, ivermectin is used to treat infestations such as head lice, scabies, river blindness (onchocerciasis), strongyloidiasis, trichuriasis, ascariasis, and lymphatic filariasis .
科学研究应用
抗寄生虫应用
伊维菌素以其抗寄生虫特性而闻名。 它被广泛用于治疗人类和动物的寄生虫感染,包括盘尾丝虫病、淋巴丝虫病和疥疮 。其功效源于其麻痹并最终杀死各种寄生虫的能力。
抗病毒研究
最近的研究探索了伊维菌素作为抗病毒药物的潜力。 它对寨卡病毒、登革热病毒和黄热病病毒等RNA病毒以及马疱疹病毒1型等DNA病毒表现出活性 。 其广谱抗病毒活性正在进一步研究,特别是在 COVID-19 大流行的背景下 。
抗癌潜力
伊维菌素已显示出对各种癌细胞系的抗增殖作用。 研究表明,它可以增强微管的稳定性,这是开发新型抗癌药物的一个有希望的途径 。它诱导癌细胞凋亡的能力是其抗癌特性的一个重要方面。
抗糖尿病研究
虽然传统上与糖尿病治疗无关,但人们对伊维菌素在抗糖尿病应用中的潜在重新利用越来越感兴趣。 其对葡萄糖代谢和胰岛素敏感性的影响是正在进行的研究领域 。
兽医学
伊维菌素是兽医学中的一个基石药物,用于治疗动物(如牛、马、羊和猪)的各种寄生虫感染 。其广谱活性使其在维护动物健康和生产力方面具有宝贵的价值。
水产养殖应用
在水产养殖中,伊维菌素用于控制鱼类种群中的寄生虫。 其在该领域的功效有助于维护养殖鱼类的健康,这对水产养殖业的可持续发展至关重要 。
环境影响研究
伊维菌素的环境影响,特别是其对土壤无脊椎动物和植物等非目标生物的毒性,是一个重要的研究领域。 了解其在环境中的归宿和影响对于制定其安全使用指南至关重要 。
药代动力学研究
伊维菌素的药代动力学特性正在研究中,以优化其使用和疗效。 对其吸收、分布、代谢和排泄的研究有助于针对不同的应用定制剂量并最大限度地减少副作用 。
作用机制
Target of Action
Ivermectin B1a, also known as Ivermectin, primarily targets glutamate-gated chloride channels . These channels are common in nematodes, insects, and ticks . The role of these channels is to regulate the flow of chloride ions across the cell membrane, which is crucial for cell function and neurotransmission .
Mode of Action
Ivermectin B1a interacts with its targets by binding to these glutamate-gated chloride channels . This binding induces irreversible channel opening, leading to a very long-lasting hyperpolarization/depolarization of the neuron/muscle cell, thereby blocking further function . This interaction disrupts normal cell function, leading to paralysis and death of the parasite .
Biochemical Pathways
It is known that the drug interferes with the normal functioning of the targeted glutamate-gated chloride channels . This interference disrupts the normal flow of chloride ions, which is essential for the regulation of electrical activity in nerve and muscle cells . The disruption of these pathways leads to paralysis and death of the parasite .
Pharmacokinetics
Ivermectin B1a is characterized by low absorption when administered subcutaneously, a large volume of distribution, very small biotransformation, and slow excretion . It has been found that the half-life of Ivermectin B1a is around a day . The kinetics of ivermectin b1a are somewhat disconnected from its pharmacodynamics, as antiparasitic events persist for several months after a single dose of the drug .
Result of Action
The primary result of Ivermectin B1a’s action is the effective treatment of various parasitic diseases. It is a broad-spectrum anti-parasitic agent, primarily deployed to combat parasitic worms in veterinary and human medicine . It is also effective against other worm-related infections and diseases, plus several parasite-induced epidermal parasitic skin diseases, as well as insect infestations .
Action Environment
The action of Ivermectin B1a can be influenced by various environmental factors. For instance, when administered to livestock, between 80 and 98% of the drug is estimated to leave the body without being metabolized in feces, thus reaching the soil . This can lead to contamination in the soil and potential harm to non-target organisms . Therefore, the prudent use of Ivermectin B1a is recommended to reduce negative effects on the environment .
生化分析
Biochemical Properties
Ivermectin B1a exerts its antiparasitic activity by binding to glutamate-gated chloride channels expressed on nematode neurons and pharyngeal muscle cells . This binding induces irreversible channel opening and very long-lasting hyperpolarization/depolarization of the neuron/muscle cell, thereby blocking further function .
Cellular Effects
Ivermectin B1a has been shown to have a broad range of effects on various types of cells. It has been reported to inhibit the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cells . In addition to its antiviral effects, Ivermectin B1a also causes immunomodulation in the host . It has been shown to increase the activity of γ-aminobutyric acid receptor or glutamate-chloride ion channel (Glu-Cl), increase the influx of chloride ions, and cause the cell membrane hyperpolarization, thereby blocking signal transmission between neurons and muscles .
Molecular Mechanism
The molecular mechanism of action of Ivermectin B1a involves binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression. Its primary target is glutamate-gated chloride channels, although it is also active against other invertebrate neurotransmitter receptors, including GABA-, histamine- and pH-sensitive channels . It produces antiparasitic activity by inducing irreversible channel opening and very long-lasting hyperpolarization/depolarization of the neuron/muscle cell .
Temporal Effects in Laboratory Settings
The effects of Ivermectin B1a over time in laboratory settings have been studied. For example, an in-vitro study reported that Ivermectin B1a inhibits SARS-CoV-2 replication with a 5000-fold reduction in viral RNA level during the first 48 h of usage . Another study found that single-dose Ivermectin B1a was ineffective in decreasing the time to a negative RT-PCR test .
Dosage Effects in Animal Models
The effects of Ivermectin B1a vary with different dosages in animal models. For example, a study of pharmacokinetics for Ivermectin B1a from Beagle dogs revealed that when the P-gp inhibitor curcumin was also co-administered orally, C max and AUC 0–∞ were found to be 177±57 and 4,213±948 h·μg·L −1, respectively .
Metabolic Pathways
Ivermectin B1a is metabolized, both in vivo and in vitro, by C-hydroxylation and O-demethylation reactions catalyzed by P450 3A4 as the major enzyme, with a contribution of P450 3A5 and 2C9 . In samples from both in vitro and in vivo metabolism, a number of metabolites were detected and as major identified metabolites were 3″-O-demethylated, C4-methyl hydroxylated, C25 isobutyl-/isopropyl-hydroxylated, and products of oxidation reactions .
Transport and Distribution
Ivermectin B1a is transported and distributed within cells and tissues. It is suggested that P-gp (MDR1) transporter participate in Ivermectin B1a efflux at low drug concentration with a slow transport rate. At the higher, micromolar concentration range, which saturates MDR1 (P-gp), MRP1, and to a lesser extent, MRP2 and MRP3 participate in Ivermectin B1a transport across physiological barriers .
Subcellular Localization
It is known that Ivermectin B1a can modulate the γ-aminobutyric acid type-A receptor (GABAAR), glycine receptor (GlyR) and neuronal α7-nicotinic receptor (nAChR), which are present in various subcellular locations .
准备方法
Ivermectin B1a is synthesized from the fermentation products of the bacterium Streptomyces avermitilis. The preparation involves several steps:
Protection of Hydroxyl Groups: The 4’'-position hydroxyl and 5
属性
| Ivermectin binds selectively and with high affinity to glutamate-gated chloride ion channels in invertebrate muscle and nerve cells of the microfilaria. This binding causes an increase in the permeability of the cell membrane to chloride ions and results in hyperpolarization of the cell, leading to paralysis and death of the parasite. Ivermectin also is believed to act as an agonist of the neurotransmitter gamma-aminobutyric acid (GABA), thereby disrupting GABA-mediated central nervous system (CNS) neurosynaptic transmission. Ivermectin may also impair normal intrauterine development of _O. volvulus_ microfilariae and may inhibit their release from the uteri of gravid female worms. | |
CAS 编号 |
70288-86-7 |
分子式 |
C48H74O14 |
分子量 |
875.1 g/mol |
IUPAC 名称 |
(1S,4S,5'S,6R,6'R,8R,10E,12S,13S,14E,16E,20R,21R,24S)-6'-[(2S)-butan-2-yl]-21,24-dihydroxy-12-[(2R,4S,5S,6S)-5-[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-4-methoxy-6-methyloxan-2-yl]oxy-5',11,13,22-tetramethylspiro[3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6,2'-oxane]-2-one |
InChI |
InChI=1S/C48H74O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38/h12-15,19,25-26,28,30-31,33-45,49-50,52H,11,16-18,20-24H2,1-10H3/b13-12+,27-15+,32-14+/t25-,26-,28-,30-,31-,33+,34-,35+,36-,37-,38-,39-,40+,41-,42-,43+,44-,45+,47+,48+/m0/s1 |
InChI 键 |
AZSNMRSAGSSBNP-TYECJXEWSA-N |
SMILES |
CCC(C)C1C(CCC2(O1)CC3CC(O2)CC=C(C(C(C=CC=C4COC5C4(C(C=C(C5O)C)C(=O)O3)O)C)OC6CC(C(C(O6)C)OC7CC(C(C(O7)C)O)OC)OC)C)C |
手性 SMILES |
CC[C@H](C)[C@@H]1[C@H](CC[C@@]2(O1)C[C@@H]3C[C@H](O2)C/C=C(/[C@H]([C@H](/C=C/C=C/4\CO[C@H]5[C@@]4([C@H](C=C([C@H]5O)C)C(=O)O3)O)C)O[C@H]6C[C@@H]([C@H]([C@@H](O6)C)O[C@H]7C[C@@H]([C@H]([C@@H](O7)C)O)OC)OC)\C)C |
规范 SMILES |
CCC(C)C1C(CCC2(O1)CC3CC(O2)CC=C(C(C(C=CC=C4COC5C4(C(C=C(C5O)C)C(=O)O3)O)C)OC6CC(C(C(O6)C)OC7CC(C(C(O7)C)O)OC)OC)C)C |
外观 |
Solid powder |
| 71827-03-7 70288-86-7 |
|
Pictograms |
Acute Toxic; Health Hazard; Environmental Hazard |
纯度 |
>90% (or refer to the Certificate of Analysis) |
保质期 |
>2 years if stored properly |
溶解度 |
Insoluble |
储存 |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
同义词 |
MK-933; Ivermectin; Ivomec; L 64047; 1 Mectizan; MK 933; MK-0933; Noromectin; Pandex. |
产品来源 |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Ivermectin exerts its antiparasitic effect by acting as an agonist for glutamate-gated chloride channels. This leads to hyperpolarization of nerve and muscle cells in invertebrates, causing paralysis and death. []
A: Unlike conventional insecticides and repellents, Ivermectin targets glutamate-gated chloride channels, disrupting nervous system function in parasites. This unique mechanism sets it apart from other mosquito control agents. []
A: Ivermectin interacts with glutamate-gated chloride channels in mammals, but with significantly lower affinity compared to invertebrates, explaining its selective toxicity. []
A: Research suggests Ivermectin may interact with other targets, including P-glycoprotein (P-gp), a transmembrane protein involved in drug efflux. This interaction could influence Ivermectin's distribution and efficacy. [, ]
A: Ivermectin treatment disrupts neuromuscular transmission in parasites, leading to paralysis, reduced microfilarial production, and ultimately, death. [, ]
A: Ivermectin (C48H74O14) has a molecular weight of 875.09 g/mol. []
A: While the provided articles don't include specific spectroscopic data, various analytical techniques like GC/MS and HPLC have been used to detect and quantify Ivermectin in biological samples. []
A: Yes, computational drug repositioning using Connectivity Map (cMap) and data/pathway mining with the Ingenuity Knowledge Base has been employed to explore Ivermectin's potential in treating gastric cancer. []
A: Computational analyses identified nine molecular targets of Ivermectin in gastric cancer, suggesting its involvement in the Wnt/β-catenin signaling pathway and cell proliferation. In silico modeling showed Ivermectin's potential to inhibit this pathway. []
A: Ivermectin's lipophilic nature presents challenges for formulation, impacting its solubility and bioavailability. Strategies to overcome these limitations might include developing novel drug delivery systems. []
A: Yes, Ivermectin is available in various formulations, including oral tablets, topical creams, and injectables for veterinary use. [, , ]
A: Ivermectin is primarily metabolized by the liver and excreted in feces. Studies in cattle have shown that after topical application, a significant portion of the drug can be ingested through licking. [, ]
A: Yes, interspecies variations in Ivermectin's pharmacokinetics have been observed, likely due to differences in factors like P-glycoprotein activity. This highlights the need for species-specific dosing regimens. []
A: Yes, a case report described an adverse interaction between Ivermectin and Warfarin, a blood thinner, leading to worsened coagulopathy. This underscores the need to consider potential drug interactions when administering Ivermectin. []
A: Following a standard oral dose of Ivermectin, peak plasma concentrations are significantly lower than the concentration required for antiviral effects observed in in vitro studies. []
A: Studies indicate that a single dose of Ivermectin can significantly reduce the transmission potential of malaria for at least two weeks. []
A: While some studies suggest a potential benefit of Ivermectin in treating COVID-19, a large randomized controlled trial (ACTIV-6) found no significant improvement in recovery time among outpatients with mild to moderate COVID-19 treated with Ivermectin compared to placebo. [, ]
A: Yes, Ivermectin effectively treats various parasitic infections, including scabies, strongyloidiasis, trichuriasis, and onchocerciasis, in both humans and animals. [, , , , ]
A: Yes, several animal models, including jirds infected with H. contortus and cattle infected with O. ochengi, have been used to evaluate Ivermectin's efficacy and investigate resistance mechanisms. [, ]
A: Yes, Ivermectin resistance has been reported in several parasite species, including H. contortus, posing a significant threat to its long-term effectiveness. [, , ]
A: While the exact mechanisms are not fully understood, mutations in glutamate-gated chloride channels and alterations in drug efflux pumps like P-glycoprotein have been implicated in Ivermectin resistance. [, , ]
A: Yes, cross-resistance between Ivermectin and other macrocyclic lactones like moxidectin has been observed in some parasite populations. []
A: Although generally well-tolerated at recommended doses, Ivermectin can cause side effects, including dizziness, nausea, and allergic reactions. Severe adverse events are rare but can occur, especially in individuals with heavy Loa loa infections. [, , , ]
A: Ivermectin toxicity has been reported in animals, particularly in certain dog breeds with a genetic mutation affecting drug metabolism. Symptoms can range from neurological signs to liver damage. [, , ]
ANone: While specific drug delivery systems weren't discussed in the provided articles, researchers are continually exploring innovative approaches to enhance drug targeting and improve Ivermectin's bioavailability.
A: Gas chromatography-mass spectrometry (GC/MS) and high-performance liquid chromatography (HPLC) are commonly employed to detect and quantify Ivermectin levels in various matrices. []
A: Ivermectin, when used topically in cattle, can contaminate the environment through fecal excretion and licking behavior. This raises concerns about potential impacts on dung-degrading insects and soil ecosystems. []
A: Ivermectin's lipophilicity can limit its dissolution in aqueous environments, potentially impacting its absorption and bioavailability. []
A: Ivermectin's interaction with P-glycoprotein, a drug efflux transporter, highlights its potential to influence drug disposition and potentially contribute to resistance mechanisms. [, ]
A: The discovery of Ivermectin by Merck scientists in 1976, followed by its development into a highly effective antiparasitic drug for both human and veterinary use, marked significant milestones in its history. [, ]
A: Programs like the Onchocerciasis Control Programme (OCP) and the African Programme for Onchocerciasis Control (APOC), involving mass drug administration of Ivermectin, have significantly reduced the burden of these diseases. []
A: Yes, research on Ivermectin resistance mechanisms in parasites has implications for understanding drug resistance in other organisms, including cancer cells. [, ]
A: The study of Ivermectin has brought together researchers from diverse fields, including parasitology, pharmacology, immunology, and computational biology, to address challenges related to its efficacy, safety, and resistance. [, , ]
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![(E)-Methyl 3-(3-(N-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)cyclohexanecarboxamido)phenyl)acrylate](/img/structure/B1672615.png)
![17-Ethynyl-17-hydroxy-7,13-dimethyl-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one](/img/structure/B1672627.png)