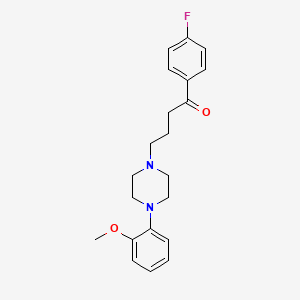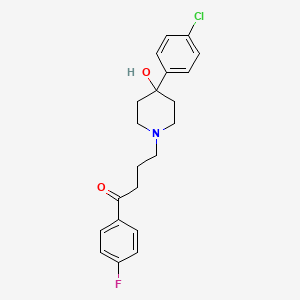
氟哌啶醇
描述
Haloperidol is a typical antipsychotic medication primarily used to treat schizophrenia, acute psychosis, and Tourette syndrome . It was discovered in 1958 by Paul Janssen and is listed on the World Health Organization’s List of Essential Medicines . Haloperidol is known for its high potency and is commonly used worldwide .
作用机制
Target of Action
Haloperidol, a high potency first-generation (typical) antipsychotic, primarily targets the dopamine receptor (mainly D2) in the brain . These receptors are particularly abundant within the mesolimbic and mesocortical systems of the brain .
Mode of Action
Haloperidol exerts its antipsychotic effect through its strong antagonism of the dopamine receptor (mainly D2) . This antagonism is thought to improve psychotic symptoms and states that are caused by an over-production of dopamine, such as schizophrenia, which is theorized to be caused by a hyperdopaminergic state within the limbic system of the brain .
Biochemical Pathways
Haloperidol is biotransformed to various metabolites, including p-fluorobenzoylpropionic acid, 4-(4-chlorophenyl)-4-hydroxypiperidine, reduced haloperidol, pyridinium metabolites, and haloperidol glucuronide . Chronic treatment with haloperidol can lead to inhibitory/excitatory imbalance in striatal D1-neurons . It has also been suggested that haloperidol may restore the levels of the proteins involved in the ubiquitin pathway in schizophrenia, possibly aiding cellular processes regulated by this pathway such as signal transduction, synaptic plasticity, intracellular trafficking, endocytosis, DNA repair, and neural activity .
Pharmacokinetics
The drug has a high protein binding capacity in humans, with around 90% bound to plasma in the blood . Haloperidol undergoes extensive metabolism in the liver, with only around 1% of the drug originally administered being excreted in the urine unchanged . The main mode of hepatic clearance is through glucuronidation, reduction, and oxidation mediated by CYP3A4 . Excretion is biliary, meaning the drug leaves the body in the feces and urine . The half-life is between 14 and 26 hours for intravenous preparations, 21 hours for intramuscular preparations, and between 14 and 37 hours for oral preparations .
Result of Action
Haloperidol’s action results in significant molecular and cellular effects. Chronic treatment with haloperidol can lead to inhibitory/excitatory imbalance in striatal D1-neurons . It has also been found to induce apoptosis and increase Caspase-8 activation in GBM cells .
Action Environment
Environmental factors can influence the action, efficacy, and stability of haloperidol. For instance, more exposures to the test environment under the influence of haloperidol cause a stronger inhibition than fewer exposures . Inflammation also seems to have a role on haloperidol pharmacokinetics .
科学研究应用
Haloperidol has a wide range of applications in scientific research:
生化分析
Biochemical Properties
Haloperidol exhibits high affinity dopamine D2 receptor antagonism and slow receptor dissociation kinetics . It binds preferentially to D2 and α1 receptors at low dose and 5-HT2 receptors at a higher dose . The enzymes involved in the biotransformation of Haloperidol include cytochrome P450 (CYP), carbonyl reductase, and uridine diphosphoglucose glucuronosyltransferase .
Cellular Effects
Haloperidol has significant effects on various types of cells and cellular processes. It influences cell function by acting as a D2 receptor antagonist, which can impact cell signaling pathways and gene expression .
Molecular Mechanism
Haloperidol exerts its effects at the molecular level primarily through its high affinity dopamine D2 receptor antagonism . This binding interaction with the D2 receptor inhibits the activity of this receptor, leading to changes in gene expression and cellular function .
Temporal Effects in Laboratory Settings
The plasma concentrations of Haloperidol reach a peak at about 6 days after injection, and its approximate half-life is 3 weeks . The concentration of Haloperidol in brain tissue is about 20-fold higher compared to blood levels . It is slowly eliminated from brain tissue, which may explain the slow disappearance of side effects when the medication is stopped .
Metabolic Pathways
Haloperidol is involved in several metabolic pathways. The greatest proportion of the intrinsic hepatic clearance of Haloperidol is by glucuronidation, followed by the reduction of Haloperidol to reduced Haloperidol and by CYP-mediated oxidation .
准备方法
Synthetic Routes and Reaction Conditions: Haloperidol is synthesized through a multi-step chemical process. The synthesis begins with the reaction of 4-chlorobenzoyl chloride with 4-fluorobutyrophenone to form 4-(4-chlorophenyl)-4-hydroxybutyrophenone. This intermediate is then reacted with piperidine to yield haloperidol .
Industrial Production Methods: Industrial production of haloperidol involves similar synthetic routes but on a larger scale. The process is optimized for high yield and purity, often involving advanced purification techniques such as recrystallization and chromatography .
化学反应分析
Types of Reactions: Haloperidol undergoes various chemical reactions, including:
Oxidation: Haloperidol can be oxidized to form haloperidol pyridinium, a metabolite.
Reduction: The carbonyl group in haloperidol can be reduced to form reduced haloperidol.
Substitution: Haloperidol can undergo substitution reactions, particularly at the piperidine ring.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and chromium trioxide.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Substitution reactions often involve nucleophiles like amines and alcohols under basic conditions.
Major Products:
Oxidation: Haloperidol pyridinium.
Reduction: Reduced haloperidol.
Substitution: Various substituted derivatives depending on the nucleophile used.
相似化合物的比较
Chlorpromazine: Known for its sedative properties but lower potency compared to haloperidol.
Fluphenazine: Similar in potency to haloperidol but with a different side effect profile.
Perphenazine: Another typical antipsychotic with a balance of potency and side effects.
Haloperidol’s unique combination of high potency and specific receptor binding profile makes it a valuable drug in the treatment of psychotic disorders, despite its side effect profile.
属性
IUPAC Name |
4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LNEPOXFFQSENCJ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CN(CCC1(C2=CC=C(C=C2)Cl)O)CCCC(=O)C3=CC=C(C=C3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H23ClFNO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4034150 | |
| Record name | Haloperidol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4034150 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
375.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Haloperidol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014645 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
23.5 [ug/mL] (The mean of the results at pH 7.4), Crystals; mp 226-227.5 °C; sol in water at 300X10+1 mg/L /Hydrochloride/, In water, 1.4X10+1 mg/L @ 25 °C, 16.7 mg/ml in alcohol at 25 °C, Freely sol in chloroform, methanol, acetone, benzene, dil acids, 4.46e-03 g/L | |
| Record name | SID855969 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Haloperidol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00502 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | HALOPERIDOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3093 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Haloperidol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014645 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
4.8X10-11 mm Hg @ 25 °C /Estimated/ | |
| Record name | HALOPERIDOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3093 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
While haloperidol has demonstrated pharmacologic activity at a number of receptors in the brain, it exerts its antipsychotic effect through its strong antagonism of the dopamine receptor (mainly D2), particularly within the mesolimbic and mesocortical systems of the brain. Schizophrenia is theorized to be caused by a hyperdopaminergic state within the limbic system of the brain. Dopamine-antagonizing medications such as haloperidol, therefore, are thought to improve psychotic symptoms by halting this over-production of dopamine. The optimal clinical efficacy of antipsychotics is associated with the blockade of approximately 60 % - 80 % of D2 receptors in the brain. While the exact mechanism is not entirely understood, haloperidol is known to inhibit the effects of dopamine and increase its turnover. Traditional antipsychotics, such as haloperidol, bind more tightly than dopamine itself to the dopamine D2 receptor, with dissociation constants that are lower than that for dopamine. It is believed that haloperidol competitively blocks post-synaptic dopamine (D2) receptors in the brain, eliminating dopamine neurotransmission and leading to the relief of delusions and hallucinations that are commonly associated with psychosis. It acts primarily on the D2-receptors and has some effect on 5-HT2 and α1-receptors, with negligible effects on dopamine D1-receptors. The drug also exerts some blockade of α-adrenergic receptors of the autonomic system. Antagonistic activity regulated through dopamine D2 receptors in the chemoreceptive trigger zone (CTZ) of the brain renders its antiemetic activity. Of the three D2-like receptors, only the D2 receptor is blocked by antipsychotic drugs in direct relation to their clinical antipsychotic abilities. Clinical brain-imaging findings show that haloperidol remains tightly bound to D2 dopamine receptors in humans undergoing 2 positron emission tomography (PET) scans with a 24h pause in between scans. A common adverse effect of this drug is the development of extrapyramidal symptoms (EPS), due to this tight binding of haloperidol to the dopamine D2 receptor. Due to the risk of unpleasant and sometimes lifelong extrapyramidal symptoms, newer antipsychotic medications than haloperidol have been discovered and formulated. Rapid dissociation of drugs from dopamine D2 receptors is a plausible explanation for the improved EPS profile of atypical antipsychotics such as [DB00734]. This is also consistent with the theory of a lower affinity for D2 receptors for these drugs. As mentioned above, haloperidol binds tightly to the dopamine receptor, potentiating the risk of extrapyramidal symptoms, and therefore should only been used when necessary., Haloperidol has less prominent autonomic effects than do other antipsychotic drugs. It has little anticholinergic activity ... it blocks activation of alpha receptors by sympathomimetic amines but is much less potent than chlorpromazine in this action., Although the complex mechanism of the therapeutic effect is not clearly established, haloperidol is known to produce a selective effect on the central nervous system (CNS) by competitive blockade of postsynaptic dopamine (D2) receptors in the mesolimbic dopaminergic system and an increased turnover of brain dopamine to produce its tranquilizing effects. With subchronic therapy, depolarization blockade, or diminished firing rate of the dopamine neuron (decreased release) along with D2 postsynaptic blockade results in the antipsychotic action. | |
| Record name | Haloperidol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00502 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | HALOPERIDOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3093 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals, WHITE TO FAINTLY YELLOWISH, AMORPHOUS OR MICRO-CRYSTALLINE POWDER | |
CAS No. |
52-86-8 | |
| Record name | Haloperidol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=52-86-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Haloperidol [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000052868 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Haloperidol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00502 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | haloperidol | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757054 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | haloperidol | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=170973 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Haloperidol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4034150 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Haloperidol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.142 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | HALOPERIDOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/J6292F8L3D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | HALOPERIDOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3093 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Haloperidol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014645 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
151.5 °C | |
| Record name | Haloperidol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00502 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | HALOPERIDOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3093 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Haloperidol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014645 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Haloperidol?
A1: Haloperidol primarily acts as an antagonist at dopamine D2 receptors. [] This means it binds to these receptors, blocking dopamine from binding and exerting its effects. [] This mechanism is believed to be central to its antipsychotic effects.
Q2: How does Haloperidol impact the ERK pathway?
A2: Haloperidol has a complex, dose- and time-dependent effect on the extracellular signal-regulated kinase (ERK) pathway, a key signaling pathway in the brain. [] It induces biphasic changes in the phosphorylation level of mitogen-activated protein kinase kinase (MEK), ERK, and p90 ribosomal S6 kinase (p90RSK), suggesting involvement of a dephosphorylating mechanism in its acute action. []
Q3: Does Haloperidol interact with other neurotransmitter systems in the brain?
A3: Research suggests Haloperidol can influence other neurotransmitter systems besides dopamine. For example, chronic haloperidol treatment in rats was shown to alter GABAA receptor density in the substantia nigra pars reticulata (SNR) and decrease dopamine D1 receptor density in the same region. [] This suggests potential interplay between dopaminergic and GABAergic systems in the context of haloperidol's effects.
Q4: Can Haloperidol be incorporated into nanoparticles for drug delivery?
A5: Yes, research demonstrates successful incorporation of Haloperidol into poly(lactic-co-glycolic acid) (PLGA) nanoparticles. [] This formulation shows promise for extended controlled release, offering potential benefits for treatment adherence and efficacy.
Q5: How do the properties of PLGA affect Haloperidol incorporation and release from nanoparticles?
A6: Studies reveal that PLGA end groups significantly influence Haloperidol encapsulation and release. [] Specifically, hydroxyl-terminated PLGA (uncapped) shows higher drug incorporation efficiency and a longer release period compared to methyl-terminated PLGA (capped). [] This highlights the importance of material selection in optimizing drug delivery systems.
Q6: How is Haloperidol metabolized in the body?
A7: Haloperidol is primarily metabolized in the liver, mainly by CYP2D6 and CYP3A4 enzymes. [, ] Variations in these enzymes' activity can impact individual responses to the drug.
Q7: Does the route of administration affect Haloperidol's pharmacokinetic profile?
A8: While all the provided studies primarily focus on oral or intramuscular Haloperidol, one study mentions that topical application of a gel containing Haloperidol does not lead to significant drug absorption. [] This suggests that different administration routes can significantly impact its bioavailability and subsequent effects.
Q8: Are there genetic factors that influence Haloperidol metabolism?
A9: Yes, genetic polymorphisms in enzymes like CYP2D6 can affect Haloperidol's pharmacokinetics. [] For instance, individuals with certain CYP2D6 genotypes might exhibit higher Haloperidol plasma concentrations compared to those without these genetic variations. []
Q9: Has Haloperidol demonstrated in vitro activity against any viruses?
A10: While one study investigated potential benefits of Haloperidol for COVID-19 based on suggested in vitro antiviral effects, the study itself did not find an association between Haloperidol use and improved clinical outcomes in hospitalized COVID-19 patients. []
Q10: What animal models have been used to study Haloperidol's effects?
A11: Several studies utilized rat models to investigate various aspects of Haloperidol's effects. These include exploring its impact on dopamine D2 receptor sensitivity, [] microglial morphology and translocator protein levels, [] and metabolic alterations in the liver. []
Q11: Are there clinical trials comparing Haloperidol to other antipsychotics?
A12: Yes, several randomized controlled trials have compared the efficacy and safety of Haloperidol to other antipsychotic medications, including atypical antipsychotics like olanzapine, risperidone, and quetiapine. [, , , , , ] These trials provide valuable insights into Haloperidol's relative effectiveness and potential advantages or disadvantages compared to other treatment options.
Q12: What are some known cardiovascular side effects associated with Haloperidol?
A13: Haloperidol can potentially cause cardiovascular side effects, with QTc prolongation, a heart rhythm abnormality, being a notable concern. [] Severe cases might lead to torsades de pointes, a life-threatening arrhythmia. []
Q13: Are there any potential long-term effects associated with Haloperidol use?
A14: One study observed that chronic Haloperidol treatment in rats led to metabolic changes in the liver, including alterations in liver mass, neutral fat deposition, and increased oxidative stress markers. [] These findings highlight the importance of monitoring for potential metabolic disturbances during long-term Haloperidol therapy.
Q14: Beyond PLGA nanoparticles, are there other potential drug delivery strategies for Haloperidol?
A14: While not explicitly explored in the provided research, other drug delivery strategies like liposomes, dendrimers, or targeted drug conjugates could potentially be investigated for Haloperidol to improve its therapeutic profile and minimize side effects.
Q15: What analytical methods are used to determine Haloperidol concentrations?
A16: High-performance liquid chromatography (HPLC) is a commonly used method to measure Haloperidol concentrations in plasma. [, ] This technique allows for accurate and sensitive quantification of the drug, aiding in dose optimization and monitoring.
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。