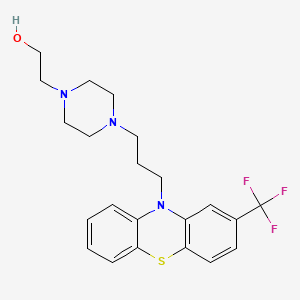
氟奋乃静
描述
氟奋乃静是一种高效典型的抗精神病药物,属于吩噻嗪类。它主要用于治疗慢性精神病,如精神分裂症。 氟奋乃静通过阻断大脑中的多巴胺受体来发挥作用,这有助于控制精神病症状 .
作用机制
氟奋乃静通过阻断大脑中突触后中脑边缘多巴胺能 D1 和 D2 受体来发挥作用。 这种作用抑制了下丘脑和垂体激素的释放,并影响网状激活系统,影响基础代谢、体温、清醒状态、血管舒缩张力和呕吐 .
类似化合物:
氯丙嗪: 另一种吩噻嗪类抗精神病药,用途类似,但效力较低。
氟哌啶醇: 一种高效抗精神病药,化学结构不同,但治疗效果相似。
氟奋乃静的独特性: 氟奋乃静的独特性在于其高效力和长效注射剂型,这提供了持续的治疗效果并提高了患者依从性 .
科学研究应用
生化分析
Biochemical Properties
Fluphenazine interacts with various biomolecules in the body. It primarily blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain . By doing so, it depresses the release of hypothalamic and hypophyseal hormones . This interaction affects basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis .
Cellular Effects
Fluphenazine has significant effects on various types of cells and cellular processes. It influences cell function by affecting cell signaling pathways, gene expression, and cellular metabolism . For instance, it can cause serious side effects, particularly movement disorders, and is known to lower people’s mood .
Molecular Mechanism
Fluphenazine exerts its effects at the molecular level through several mechanisms. It blocks dopamine receptors, primarily D1 and D2 . This blockade depresses the release of hypothalamic and hypophyseal hormones, which in turn affects various physiological processes .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Fluphenazine can change over time. For instance, in rat models, Fluphenazine and its metabolites were detected in tissues at higher levels than in plasma, and the levels increased with dose .
Dosage Effects in Animal Models
In animal models, the effects of Fluphenazine vary with different dosages. For example, systemic Fluphenazine effectively attenuated mechanical allodynia in rat neuropathic pain models at doses (0.03–0.3 mg/kg) that approximate those used in rodent models of psychosis .
Metabolic Pathways
Fluphenazine is involved in various metabolic pathways. The metabolism of Fluphenazine is complex. It is metabolized extensively by aromatic hydroxylation, S- and N-oxidation, N-desalkylation, and glucuronidation .
Transport and Distribution
Fluphenazine is transported and distributed within cells and tissues. In rat models, Fluphenazine was found to be 10- to 27-fold higher in brain regions than in plasma . Liver contained the highest levels of all analytes at all doses .
Subcellular Localization
It is known that Fluphenazine and its metabolites are detected in tissues at higher levels than in plasma, indicating that it may be localized in specific subcellular compartments .
准备方法
合成路线和反应条件: 氟奋乃静是通过多步合成过程合成的。合成始于2-三氟甲基吩噻嗪的制备,这是通过从氟芬那酸开始的脱羧和环化反应实现的。然后将该中间体与1-(2-乙氧基)哌嗪和1,3-溴氯丙烷反应生成1-(3-氯丙基)-4-(2-乙氧基)哌嗪。 最后,这些中间体之间的缩合反应产生了氟奋乃静 .
工业生产方法: 氟奋乃静的工业生产涉及类似的合成路线,但规模更大。 该工艺针对产率和纯度进行了优化,通常涉及高效液相色谱 (HPLC) 用于质量控制 .
化学反应分析
反应类型: 氟奋乃静会发生各种化学反应,包括:
氧化: 氟奋乃静在特定条件下可以被氧化,导致降解产物的形成。
还原: 还原反应可以改变氟奋乃静中的官能团,改变其药理特性。
常用试剂和条件:
氧化: 过氧化氢或其他氧化剂在受控条件下。
还原: 还原剂如氢化铝锂。
取代: 卤化剂用于引入或修饰卤素基团。
相似化合物的比较
Chlorpromazine: Another phenothiazine antipsychotic with similar uses but lower potency.
Haloperidol: A high-potency antipsychotic with a different chemical structure but similar therapeutic effects.
Perphenazine: A phenothiazine antipsychotic with comparable efficacy but different side effect profiles
Uniqueness of Fluphenazine: Fluphenazine is unique due to its high potency and long-acting injectable formulations, which provide sustained therapeutic effects and improve patient compliance .
属性
IUPAC Name |
2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]propyl]piperazin-1-yl]ethanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PLDUPXSUYLZYBN-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CN(CCN1CCCN2C3=CC=CC=C3SC4=C2C=C(C=C4)C(F)(F)F)CCO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H26F3N3OS | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2023068 | |
| Record name | Fluphenazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023068 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
437.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Fluphenazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014761 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
268-274 °C at 5.00E-01 mm Hg, BP: 250-252 °C at 0.3 mm Hg; 268-274 °C at 0.5 mm Hg | |
| Record name | Fluphenazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00623 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluphenazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3334 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
In water, 31.1 g/L (31.3 mg/L) at 37 °C, 1.90e-02 g/L | |
| Record name | Fluphenazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00623 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluphenazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3334 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Fluphenazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014761 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Fluphenazine blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis., Agranulocytosis and the release of transaminase enzymes from liver cells are known consequences of neuroleptic drug use. These effects are most common with low potency neuroleptic drugs. It has been hypothesized that these effects are due to the direct toxic action of these drugs on blood and liver cells. The purpose of this study is to compare the cytotoxic effects of eight neuroleptic drugs in five different biological test systems. In all of the test systems, thioridazine, chlorpromazine, trifluoperazine, fluphenzine and thiothixene (group one drugs) were the most toxic drugs and molindone was the least toxic. Thioridazine was between 25 and 84 times more toxic than molindone. Loxapine was significantly more toxic than molindone, but less toxic than the group one drugs. Haloperidol was intermediate in toxicity between the group one drugs and loxapine. /It was/ concluded that the difference in cytotoxicity of the neuroleptic drugs observed in these experiments accounts in part for the increase in agranulocytosis and hepatotoxicity with thioridazine and chlorpromazine and for the lower incidence of these side effects with less toxic drugs. The possibility that tardive dyskinesia may be due to the cytotoxic effects of neuroleptic drugs is discussed and an experiment to test this hypothesis is suggested., The principal pharmacologic effects of fluphenazine are similar to those of chlorpromazine. Fluphenazine is more potent on a weight basis than chlorpromazine. Fluphenazine has weak anticholinergic and sedative effects and strong extrapyramidal effects. Fluphenazine has weak antiemetic activity., The development of phenothiazine derivatives as psychopharmacologic agents resulted from the observation that certain phenothiazine antihistaminic compounds produced sedation. In an attempt to enhance the sedative effects of these drugs, promethazine and chlorpromazine were synthesized. Chlorpromazine is the pharmacologic prototype of the phenothiazines. The pharmacology of phenothiazines is complex, and because of their actions on the central and autonomic nervous systems, the drugs affect many different sites in the body. Although the actions of the various phenothiazines are generally similar, these drugs differ both quantitatively and qualitatively in the extent to which they produce specific pharmacologic effects. /Phenothiazine General Statement/, In the CNS, phenothiazines act principally at the subcortical levels of the reticular formation, limbic system, and hypothalamus. Phenothiazines generally do not produce substantial cortical depression; however, there is minimal information on the specific effects of phenothiazines at the cortical level. Phenothiazines also act in the basal ganglia, exhibiting extrapyramidal effects. The precise mechanism(s) of action, including antipsychotic action, of phenothiazines has not been determined, but may be principally related to antidopaminergic effects of the drugs. There is evidence to indicate that phenothiazines antagonize dopamine-mediated neurotransmission at the synapses. There is also some evidence that phenothiazines may block postsynaptic dopamine receptor sites. However, it has not been determined whether the antipsychotic effect of the drugs is causally related to their antidopaminergic effects. Phenothiazines also have peripheral and/or central antagonistic activity against alpha-adrenergic, serotonergic, histaminic (H1-receptors), and muscarinic receptors. Phenothiazines also have some adrenergic activity, since they block the reuptake of monoamines at the presynaptic neuronal membrane, which tends to enhance neurotransmission. The effects of phenothiazines on the autonomic nervous system are complex and unpredictable because the drugs exhibit varying degrees of alpha-adrenergic blocking, muscarinic blocking, and adrenergic activity. The antipsychotic activity of phenothiazines may be related to any or all of these effects, but it has been suggested that the drugs' effects on dopamine are probably most important. It has also been suggested that effects of phenothiazines on other amines (eg, gamma-aminobutyric acid [GABA]) or peptides (eg, substance P, endorphins) may contribute to their antipsychotic effect. Further study is needed to determine the role of central neuronal receptor antagonism and of effects on biochemical mediators in the antipsychotic action of the phenothiazines and other antipsychotic agents. /Phenothiazine General Statement/, For more Mechanism of Action (Complete) data for Fluphenazine (17 total), please visit the HSDB record page. | |
| Record name | Fluphenazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00623 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluphenazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3334 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Dark brown viscous oil | |
CAS No. |
69-23-8 | |
| Record name | Fluphenazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=69-23-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Fluphenazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000069238 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Fluphenazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00623 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluphenazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023068 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Fluphenazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.639 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | FLUPHENAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/S79426A41Z | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Fluphenazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3334 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Fluphenazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014761 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
224-226 (Salt), Crystals from absolute alcohol. MP: 235-237 °C, also reported as 224.5-226 °C /Fluphenazine dihydrochloride/, Pale yellow-orange, viscous liquid. Slowly crystallizes at room temperature. MP: 30-32 °C. Very soluble in chloroform, ether, cyclohexane, methanol, ethanol. Insoluble in water /Fluphenazine decanoate/, < 25 °C | |
| Record name | Fluphenazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00623 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluphenazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3334 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Fluphenazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014761 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Fluphenazine?
A1: Fluphenazine primarily exerts its antipsychotic effects by acting as a dopamine antagonist. It binds to and blocks dopamine D2 receptors in the brain, particularly in the mesolimbic and mesocortical pathways [, , , , ].
Q2: How does Fluphenazine's dopamine antagonism relate to its efficacy in treating schizophrenia?
A2: The dopamine hypothesis of schizophrenia proposes that an overactive dopamine system in certain brain regions contributes to the positive symptoms of the disorder, such as hallucinations and delusions. By blocking D2 receptors, Fluphenazine helps to reduce dopamine signaling in these areas, thereby alleviating these symptoms [, , , ].
Q3: Can chronic Fluphenazine administration lead to changes in dopamine receptor sensitivity?
A3: Research suggests that long-term treatment with dopamine antagonists, including Fluphenazine, can lead to adaptive changes in dopamine receptor sensitivity. Studies in rats have shown that chronic Fluphenazine administration can result in enhanced sensitivity of both central and peripheral dopamine receptors [].
Q4: What is the molecular formula and weight of Fluphenazine?
A4: The molecular formula of Fluphenazine is C22H27F3N3OS+Cl−, and its molecular weight is 437.96 g/mol.
Q5: Are there different forms or esters of Fluphenazine available?
A5: Yes, Fluphenazine is available in different ester forms, including Fluphenazine decanoate and Fluphenazine enanthate. These esters are formulated as long-acting injectables (LAIs), which provide sustained release of the medication over several weeks [, , ].
Q6: How do the pharmacokinetic properties of Fluphenazine decanoate and Fluphenazine enanthate differ?
A6: While both Fluphenazine decanoate and Fluphenazine enanthate are LAIs, they differ in their pharmacokinetic profiles. Fluphenazine decanoate generally has a longer duration of action compared to Fluphenazine enanthate [, ]. Studies have shown that similar plasma levels of Fluphenazine are achieved with doses of Fluphenazine enanthate that are twice those of Fluphenazine decanoate [].
Q7: Has the metabolic fate of Fluphenazine been investigated?
A7: Yes, research has identified several metabolites of Fluphenazine, including Fluphenazine sulfoxide, 7-hydroxyfluphenazine, and Fluphenazine N4’-oxide [, ]. These metabolites have been detected in various biological samples, including plasma, urine, and bile [, , ].
Q8: How does the stability of Fluphenazine impact its formulation and administration?
A8: Fluphenazine, particularly in its oral formulations, can be susceptible to degradation. To enhance its stability and bioavailability, various formulation strategies have been explored, including the development of LAIs [, ].
Q9: What are the advantages of using long-acting injectable formulations of Fluphenazine?
A9: LAIs of Fluphenazine offer several potential advantages, including improved adherence to medication, reduced risk of relapse, and more consistent plasma drug levels [, ]. These formulations are particularly beneficial for individuals who struggle with adherence to daily oral medications.
Q10: What are some of the known adverse effects associated with Fluphenazine use?
A10: Fluphenazine, like other typical antipsychotics, can cause extrapyramidal side effects (EPS) such as akathisia, rigidity, and tardive dyskinesia. Research suggests that these effects may be related to Fluphenazine's dopamine antagonism in the nigrostriatal pathway, which is involved in motor control [, , , ].
Q11: Are there any potential cardiovascular risks associated with Fluphenazine?
A11: While not as common as EPS, Fluphenazine has been associated with cardiovascular adverse effects, including cardiac conduction abnormalities. These effects are thought to be related to Fluphenazine's ability to prolong the QT interval on an electrocardiogram [].
Q12: How do the adverse effect profiles of Fluphenazine and atypical antipsychotics compare?
A12: While Fluphenazine is considered an effective antipsychotic, its use is often limited by its potential for EPS. Atypical antipsychotics are generally associated with a lower risk of EPS, although they may have other side effects [].
Q13: What analytical techniques are commonly employed for the detection and quantification of Fluphenazine?
A13: Various analytical methods have been developed for Fluphenazine analysis, including spectrophotometry, high-performance liquid chromatography (HPLC), and gas chromatography-mass spectrometry (GC-MS) [, , ].
Q14: What are the key considerations for the development and validation of analytical methods for Fluphenazine?
A14: Analytical methods for Fluphenazine must be accurate, precise, specific, and robust. Method validation ensures that the method is fit for its intended purpose and provides reliable results [, ].
Q15: What is the role of clinical trials in evaluating the efficacy and safety of Fluphenazine?
A15: Clinical trials are essential for rigorously evaluating the efficacy, safety, and tolerability of Fluphenazine in treating schizophrenia. Randomized controlled trials (RCTs) are considered the gold standard for determining the efficacy of a medication compared to placebo or other treatments [, , , , ].
Q16: Have there been any comparative clinical trials evaluating Fluphenazine against other antipsychotic medications?
A16: Yes, several RCTs have compared Fluphenazine with other antipsychotic medications, both typical and atypical. These trials have provided valuable insights into the relative efficacy, tolerability, and safety profiles of different antipsychotic options [, , , ].
Q17: Have any biomarkers been identified that can predict response to Fluphenazine treatment?
A17: Research on biomarkers for predicting Fluphenazine response is ongoing. Some studies have suggested a potential role for cerebrospinal fluid (CSF) biomarkers, such as the ratio of homovanillic acid (HVA) to 5-hydroxyindoleacetic acid (5-HIAA), in predicting response to clozapine, another antipsychotic medication, in individuals with treatment-resistant schizophrenia [].
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![(2S,3R,4S,5S,6R)-2-[(2E)-3,7-dimethylocta-2,6-dienoxy]-6-[[(2S,3R,4S,5S)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxane-3,4,5-triol](/img/structure/B1673392.png)
![(2S)-2-[[(2S)-2-aminohexanoyl]amino]-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[(2-amino-2-oxoethyl)amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(4H-imidazol-4-yl)-1-oxo](/img/structure/B1673405.png)
![N-[(1R,3S)-3-Hydroxy-1-(hydroxymethyl)-3-phenylpropyl]dodecanamide](/img/structure/B1673406.png)
![N-[(3-Hydroxypyridin-2-Yl)carbonyl]glycine](/img/structure/B1673407.png)
![N-[(3-Hydroxy-2-quinolinyl)carbonyl]-glycine](/img/structure/B1673411.png)
![3-[4-(8-fluoro-5H-[1]benzoxepino[4,3-b]pyridin-11-ylidene)piperidin-1-yl]propanoic acid](/img/structure/B1673414.png)
