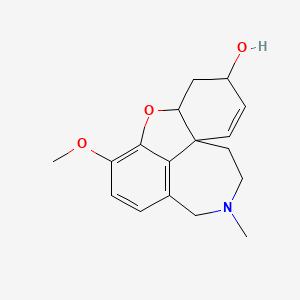
加兰他敏
概述
描述
Galantamine: is a natural alkaloid extracted from the bulbs and flowers of various plants in the Amaryllidaceae family, such as Galanthus nivalis (common snowdrop), Galanthus caucasicus (Caucasian snowdrop), and others . It is primarily known for its potential to slow cognitive decline and is used clinically for treating early-stage Alzheimer’s disease and memory impairments .
作用机制
Target of Action
Galanthamine, also known as Galantamine, primarily targets the acetylcholinesterase (AChE) enzyme . AChE is a widely studied therapeutic target used in the treatment of Alzheimer’s disease . Galanthamine acts as a reversible, competitive inhibitor of AChE . It also acts as an allosteric modulator of the nicotinic receptor , giving its dual mechanism of action clinical significance .
Mode of Action
Galanthamine works by blocking the breakdown of acetylcholine in the synaptic cleft, thereby increasing acetylcholine neurotransmission . This is achieved by inhibiting the activity of the AChE enzyme, which is responsible for the hydrolysis of acetylcholine . By slowing down acetylcholine catabolism, galanthamine increases the levels of acetylcholine in the synaptic cleft .
Biochemical Pathways
The primary biochemical pathway affected by galanthamine is the cholinergic pathway . By inhibiting AChE, galanthamine increases the concentration of acetylcholine, a key neurotransmitter in this pathway . This leads to enhanced cholinergic neuron function and signaling .
Pharmacokinetics
Galanthamine exhibits good bioavailability, with 80–100% of the drug being absorbed after oral administration . It is partially metabolized in the liver by the CYP2D6 and CYP3A4 enzymes . The elimination half-life of galanthamine is approximately 7 hours . About 95% of the drug is excreted in the urine, with 32% of this being unchanged . The remaining 5% is excreted in the feces .
Result of Action
The primary molecular effect of galanthamine’s action is the increased concentration of acetylcholine in the synaptic cleft . This leads to enhanced cholinergic neuron function and signaling . On a cellular level, this results in improved cognitive function, including memory processing, reasoning, and thinking .
Action Environment
Environmental factors can influence the action, efficacy, and stability of galanthamine. For instance, studies have shown that water stress conditions can affect the bioaccumulation of galanthamine in plants . Moderate water deficiency (50% water deficit irrigation) was found to produce the highest levels of galanthamine . This suggests that environmental conditions can play a significant role in the availability and efficacy of galanthamine.
科学研究应用
Chemistry: Galantamine is used as a model compound in synthetic organic chemistry to study complex natural product synthesis .
Biology: In biological research, galantamine is used to study the mechanisms of acetylcholinesterase inhibition and its effects on neurotransmission .
Medicine: Galantamine is clinically used to manage mild to moderate dementia associated with Alzheimer’s disease . It is also being investigated for its potential use in other neurodegenerative disorders .
Industry: In the pharmaceutical industry, galantamine is used as an active ingredient in drugs for treating cognitive impairments .
生化分析
Biochemical Properties
Galanthamine inhibits the degradation of neurotransmitters acetylcholine (ACh) by binding to the enzyme, acetylcholinesterase (AChE) which is responsible for degrading ACh . The resulting accumulation of ACh caused by galanthamine leads to increased neurotransmission causing continuous stimulation of the muscles, glands .
Cellular Effects
The increased neurotransmission caused by galanthamine can have various effects on cells. It can influence cell function by impacting cell signaling pathways and gene expression. It can also affect cellular metabolism by altering the levels of neurotransmitters in the cell .
Molecular Mechanism
Galanthamine exerts its effects at the molecular level through its binding interactions with acetylcholinesterase. By inhibiting this enzyme, galanthamine prevents the degradation of acetylcholine, leading to an increase in the levels of this neurotransmitter. This can result in changes in gene expression and cellular function .
Temporal Effects in Laboratory Settings
The effects of galanthamine can change over time in laboratory settings. Information on the product’s stability, degradation, and any long-term effects on cellular function observed in in vitro or in vivo studies is currently being researched .
Dosage Effects in Animal Models
The effects of galanthamine can vary with different dosages in animal models. Studies are being conducted to determine any threshold effects observed in these studies, as well as any toxic or adverse effects at high doses .
Metabolic Pathways
Galanthamine is involved in various metabolic pathways. It interacts with enzymes such as acetylcholinesterase and can affect metabolic flux or metabolite levels .
Transport and Distribution
Research is ongoing to determine how galanthamine is transported and distributed within cells and tissues. This includes studying any transporters or binding proteins that it interacts with, as well as any effects on its localization or accumulation .
Subcellular Localization
The subcellular localization of galanthamine and any effects on its activity or function are currently being researched. This includes studying any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
准备方法
Synthetic Routes and Reaction Conditions: Galantamine can be synthesized through various methods, including biomimetic oxidative coupling reactions, transition metal-catalyzed reactions, and rearrangement reactions . Some key synthetic strategies include:
Oxidative Phenol Coupling: This method involves the oxidative coupling of phenols, which has been extensively studied for the total synthesis of galantamine.
Transition Metal-Catalyzed Reactions: These include the Heck reaction, enyne ring-closing metathesis (RCM), and dynamic kinetic resolution.
Rearrangement Reactions: Examples include the semipinacol rearrangement and the Johnson–Claisen rearrangement.
Industrial Production Methods: The industrial production of galantamine involves the extraction from natural sources or synthetic production. The first industrial process was developed in 1959 . Recent advances have focused on optimizing synthetic routes to improve yield and reduce costs .
化学反应分析
Types of Reactions: Galantamine undergoes various chemical reactions, including:
Oxidation: Galantamine can be oxidized to form various derivatives.
Reduction: Reduction reactions can modify the functional groups in galantamine.
Substitution: Substitution reactions can introduce different functional groups into the galantamine molecule.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Various reagents, including halogens and alkylating agents, are used for substitution reactions.
Major Products: The major products formed from these reactions include various galantamine derivatives with modified functional groups, which can have different pharmacological properties .
相似化合物的比较
Rivastigmine: Another acetylcholinesterase inhibitor used for treating Alzheimer’s disease.
Donepezil: A widely used acetylcholinesterase inhibitor with a similar mechanism of action.
Lycorine: An alkaloid with acetylcholinesterase inhibitory activity, though less potent than galantamine.
Uniqueness: Galantamine is unique due to its dual mechanism of action, both inhibiting acetylcholinesterase and modulating nicotinic acetylcholine receptors . This dual action enhances its therapeutic efficacy compared to other acetylcholinesterase inhibitors .
属性
IUPAC Name |
(1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ASUTZQLVASHGKV-JDFRZJQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCC23C=CC(CC2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CC[C@@]23C=C[C@@H](C[C@@H]2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H21NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2045606 | |
| Record name | Galanthamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2045606 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
287.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Crystals from water; decomposition 256-257 °C. Sparingly sol in cold; more sol in hot water. Very sparingly sol in alcohol, acetone. /Hydrochloride/, Fairly soluble in hot water; freely soluble in alcohol, acetone, chloroform. Less sol in benzene, ether., 1.70e+00 g/L | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Alzheimer’s disease is characterized by progressive, irreversible degeneration of acetylcholine-producing neurons, cognitive impairment, and the accumulation of neurofibrillary tangles and amyloid plaques. The cholinergic system plays a critical role in memory, alongside other important neural functions such as attention, learning, stress response, wakefulness and sleep, and sensory information. Studies show that acetylcholine (ACh) is involved in the modulation of acquisition, encoding, consolidation, reconsolidation, extinction, and retrieval of memory. The gradual loss of cholinergic neurons in Alzheimer’s disease (AD) may, therefore, contribute to the memory loss exhibited by AD patients. Acetylcholinesterase is secreted by cholinergic neurons to rapidly hydrolyze ACh at the synaptic cleft to release acetate and choline. Choline is later recycled back into the presynaptic cholinergic neuron via reuptake by the high-affinity choline transporter. There is some evidence demonstrating the potential involvement of the acetylcholinesterase enzyme in the formation of amyloid fibrils. Galantamine competitively and reversibly inhibits the anticholinesterase enzyme in the CNS (namely in the frontal cortex and hippocampal regions) by binding to the choline-binding site and acyl-binding pocket of the enzyme active site. By blocking the breakdown of ACh, galantamine enhances ACh levels in the synaptic cleft. Nicotinic acetylcholine receptors (nAChR) in the CNS are mostly expressed at the presynaptic neuronal membrane to control the release of multiple neurotransmitters, such as ACh, glutamate, GABA, dopamine, serotonin, norepinephrine. Agonists of nAChRs improve performance in cognitive tasks, while antagonists of nAChR impair cognitive processes. Some studies show a decrease in the expression and activity of nAChRs in patients with AD, which may explain the reduction in central cholinergic neurotransmission in these patients. Galantamine binds to nAChRs at the allosteric site, leading to a conformational change of the receptor, increased ACh release, and increased activity of neighbouring glutaminergic and serotoninergic neurons. The modulation of nAChRs facilitates both excitatory and inhibitory cholinergic transmissions in brain tissues and increases receptor sensitivity. The modulated release of other neurotransmitters by galantamine may also contribute to the upregulation of nAChRs and amelioration of behavioural symptoms in AD., Galantamine, a tertiary alkaloid, is a competitive and reversible inhibitor of acetylcholinesterase. While the precise mechanism of galantamine's action is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this mechanism is correct, galantamine's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that galantamine alters the course of the underlying dementing process., The cause of cognitive impairment in Alzheimer's Disease is not fully understood, it has been shown that acetylcholine producing neurons degenerate. The cholinergic loss has been correlated with cognitive impairment and a density of amyloid plaques. Galantamine is a tertiary alkaloid and it competes with and is a reversible inhibitor of acetylcholinesterase. The exact mechanism of galantamine is not known, but it is believed to enhance cholinergic function. | |
| Record name | Galantamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00674 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from benzene | |
CAS No. |
357-70-0, 23173-12-8 | |
| Record name | (-)-Galantamine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=357-70-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Galantamine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000357700 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (+/-)-Galantamine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023173128 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Galantamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00674 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Galantamine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759861 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Galanthamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2045606 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | GALANTAMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0D3Q044KCA | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | GALANTAMINE, (±)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1T835Z585R | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
126-127 °C, 269 - 270 °C (hydrogen bromide salt) | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details












Synthesis routes and methods II
Procedure details











Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。
![[(1s)-1-Isothiocyanatoethyl]benzene](/img/structure/B1674335.png)