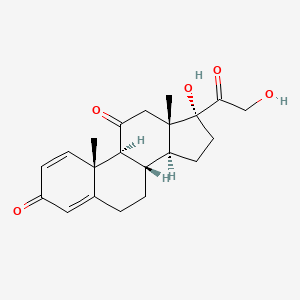
泼尼松
作用机制
泼尼松通过在肝脏中转化为泼尼松龙而发挥作用 . 然后,泼尼松龙与糖皮质激素受体结合,激活它们并触发基因表达的变化 . 这导致抑制多形核白细胞的迁移和逆转毛细血管通透性增加,从而减少炎症 . 它还通过降低免疫系统的活性和体积来抑制免疫系统 .
类似化合物:
- 地塞米松
- 甲氨蝶呤
- 霉酚酸
- 巯嘌呤
- 硫唑嘌呤
- 来氟米特
比较: 泼尼松独特的特点是可以转化为活性形式泼尼松龙,后者具有强大的抗炎和免疫抑制作用 . 与地塞米松相比,泼尼松的半衰期更短,效力更低 . 甲氨蝶呤和霉酚酸主要用作免疫抑制剂,但没有泼尼松相同的抗炎特性 . 巯嘌呤、硫唑嘌呤和来氟米特也用作免疫抑制剂,但它们的作用机制不同,并在不同的临床环境中使用 .
科学研究应用
生化分析
Biochemical Properties
Prednisone interacts with various enzymes, proteins, and other biomolecules. It is metabolized to its active form, prednisolone, in the liver through the 11-hydroxysteroid dehydrogenase enzyme (HSD11B1) . Prednisolone then binds to glucocorticoid receptors, activating them and triggering changes in gene expression .
Cellular Effects
Prednisone influences cell function by suppressing virtually every component of the inflammatory process . It inhibits the synthesis of interleukins and numerous other proinflammatory cytokines, suppresses cell-mediated immunity, reduces complement synthesis, and decreases production and activity of leukocytes .
Molecular Mechanism
Prednisone exerts its effects at the molecular level through binding interactions with biomolecules and changes in gene expression. Prednisone is a prodrug and must be converted to prednisolone by the liver before it becomes active . Prednisolone then binds to glucocorticoid receptors, activating them and triggering changes in gene expression .
Temporal Effects in Laboratory Settings
The effects of prednisone change over time in laboratory settings. For example, a study showed that even short-term, low dosage use of prednisone was associated with higher rates of sepsis, venous thromboembolism, and fractures within 30 days of starting on steroids .
Dosage Effects in Animal Models
The effects of prednisone vary with different dosages in animal models. A study on dogs showed that prednisone concentrations in various tissues were increased or decreased depending on the dosage and the specific tissue .
Metabolic Pathways
Prednisone is involved in various metabolic pathways. It is converted to its active metabolite prednisolone in the liver through the 11-hydroxysteroid dehydrogenase enzyme (HSD11B1) . Prednisolone is further metabolized primarily via cytochrome P450 enzyme CYP3A (CYP3A4 and possibly CYP3A5) .
Transport and Distribution
Prednisone is transported and distributed within cells and tissues. A study showed that the tissue distribution of prednisone and prednisolone was affected by certain factors, leading to increased or decreased concentrations in various tissues .
Subcellular Localization
The subcellular localization of prednisone and its effects on its activity or function are complex. Prednisone is a prodrug and must be converted to prednisolone by the liver before it becomes active . Prednisolone then binds to glucocorticoid receptors, which are located in the cytoplasm of cells, and the receptor-ligand complex then translocates to the nucleus, where it regulates gene expression .
准备方法
合成路线和反应条件: 泼尼松的制备通常涉及从霉菌氧化物开始的几个步骤。 该过程包括 Platt 氧化反应,然后是溴加成反应、溴去除和 21 位碘加成反应 . 最后一步涉及 21 位取代反应以获得泼尼松 .
工业生产方法: 泼尼松的工业生产通常涉及使用醋酸氢化可的松作为起始原料 . 该过程包括氧化、还原和酯化等步骤,以大规模生产泼尼松 . 这些方法旨在通过回收铬和乙酸来最大限度地减少浪费并减少对环境的影响 .
化学反应分析
反应类型: 泼尼松会经历各种化学反应,包括氧化、还原和取代 .
常见试剂和条件:
氧化: 使用霉菌氧化物进行 Platt 氧化反应.
还原: 还原反应通常涉及使用特定的还原剂将中间体化合物转化为泼尼松.
取代: 泼尼松合成中常见溴加成和去除反应.
相似化合物的比较
- Dexamethasone
- Methotrexate
- Mycophenolate
- Mercaptopurine
- Azathioprine
- Leflunomide
Comparison: Prednisone is unique in its ability to be converted to an active form, prednisolone, which has potent anti-inflammatory and immunosuppressive effects . Compared to dexamethasone, prednisone has a shorter half-life and is less potent . Methotrexate and mycophenolate are primarily used as immunosuppressants but do not have the same anti-inflammatory properties as prednisone . Mercaptopurine, azathioprine, and leflunomide are also used as immunosuppressants but have different mechanisms of action and are used in different clinical contexts .
属性
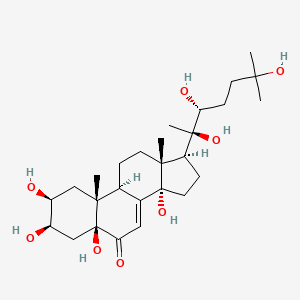
IUPAC Name |
(8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,12,14,15,16-octahydrocyclopenta[a]phenanthrene-3,11-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H26O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-15,18,22,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XOFYZVNMUHMLCC-ZPOLXVRWSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC12CC(=O)C3C(C1CCC2(C(=O)CO)O)CCC4=CC(=O)C=CC34C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@]12CC(=O)[C@H]3[C@H]([C@@H]1CC[C@@]2(C(=O)CO)O)CCC4=CC(=O)C=C[C@]34C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H26O5 | |
| Record name | PREDNISONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20949 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4021185 | |
| Record name | Prednisone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4021185 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
358.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Prednisone is an odorless white crystalline powder. (NTP, 1992), Solid | |
| Record name | PREDNISONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20949 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Prednisone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014773 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Very slightly soluble (NTP, 1992), Very slightly soluble, Very slightly soluble in water; 1 g soluble in 150 mL alcohol, in 200 mL chloroform; slightly soluble in methanol, 1.11e-01 g/L | |
| Record name | PREDNISONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20949 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Prednisone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00635 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PREDNISONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3168 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Prednisone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014773 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Prednisone is first metabolized in the liver to its active form, prednisolone, a glucocorticoid agonist corticosteroid. The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels., In physiologic doses, corticosteroids are administered to replace deficient endogenous hormones. In larger (pharmacologic) doses, glucocorticoids decrease inflammation by stabilizing leukocyte lysosomal membranes, preventing release of destructive acid hydrolases from leukocytes; inhibiting macrophage accumulation in inflamed areas; reducing leukocyte adhesion to capillary endothelium; reducing capillary wall permeability and edema formation; decreasing complement components; antagonizing histamine activity and release of kinin from substrates; reducing fibroblast proliferation, collagen deposition, and subsequent scar tissue formation; and possibly by other mechanisms as yet unknown. The drugs suppress the immune response by reducing activity and volume of the lymphatic system, producing lymphocytopenia, decreasing immunoglobulin and complement concentrations, decreasing passage of immune complexes through basement membranes, and possibly by depressing reactivity of tissue to antigen-antibody interactions. Glucocorticoids stimulate erythroid cells of bone marrow, prolong survival time of erythrocytes and platelets, and produce neutrophilia and eosinopenia. Glucocorticoids promote gluconeogenesis, redistribution of fat from peripheral to central areas of the body, and protein catabolism, which results in negative nitrogen balance. They reduce intestinal absorption and increase renal excretion of calcium. /Corticosteroids/, Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/ | |
| Record name | Prednisone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00635 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PREDNISONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3168 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals, White to practically white, crystalline powder | |
CAS No. |
53-03-2 | |
| Record name | PREDNISONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20949 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Prednisone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=53-03-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Prednisone [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000053032 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Prednisone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00635 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | prednisone | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=10023 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pregna-1,4-diene-3,11,20-trione, 17,21-dihydroxy- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Prednisone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4021185 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Prednisone | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.147 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PREDNISONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/VB0R961HZT | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PREDNISONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3168 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Prednisone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014773 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
451 to 455 °F (DEC) (NTP, 1992), 234 °C (decomposes), 233 - 235 °C | |
| Record name | PREDNISONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20949 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Prednisone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00635 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PREDNISONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3168 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Prednisone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014773 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of prednisone?
A1: Prednisone itself is a prodrug that is converted to its active metabolite, prednisolone, in the liver. Prednisolone acts by binding to the glucocorticoid receptor (GR) in the cytoplasm. [] This complex then translocates to the nucleus and influences gene expression, ultimately leading to a wide range of anti-inflammatory and immunosuppressive effects. []
Q2: How does prednisone differ from prednisolone in terms of activity?
A2: Prednisone is inactive until it is metabolized into prednisolone in the liver. [, ] Individuals lacking the necessary hepatic enzyme system may exhibit reduced clinical effects from prednisone. []
Q3: Can you elaborate on the immunosuppressive effects of prednisone?
A3: Prednisone, via prednisolone, exerts its immunosuppressive effects by modulating various immune cell functions. For instance, it inhibits interleukin 2 (IL-2) production and alters the ratio of helper T cells (OKT4+) to suppressor T cells (OKT8+). [] This modulation of cytokine production and immune cell populations contributes to its immunosuppressive properties. [, , ]
Q4: A study mentioned that prednisone slows the deterioration of muscle function in Duchenne Muscular Dystrophy (DMD). How does this effect relate to its primary mechanism of action?
A4: While the exact mechanism remains unclear, it's suggested that prednisone's anti-inflammatory properties might play a role in reducing muscle inflammation in DMD, thereby slowing down muscle fiber degeneration. []
Q5: What is the molecular formula and weight of prednisone?
A5: The molecular formula of prednisone is C21H26O5, and its molecular weight is 358.43 g/mol. [, ]
Q6: Are there any specific spectroscopic techniques used to characterize prednisone?
A6: Researchers utilize various techniques, including UV spectrophotometry, to characterize and quantify prednisone. [] For instance, UV spectrophotometry was employed to determine prednisone concentrations during the development of a biodegradable microsphere formulation. []
Q7: Has prednisone been formulated into a long-term controlled-release system?
A7: Yes, researchers have successfully developed prednisone-loaded biodegradable microspheres using poly(DL-lactide-co-glycolide) (PLGA) polymers. [] These microspheres demonstrate a suitable size and exhibit a long-term release profile. []
Q8: How is prednisone absorbed and distributed in the body?
A8: Prednisone is administered orally and absorbed from the gastrointestinal tract. [, ] It is then distributed to various tissues, though it does not readily cross the blood-brain barrier. []
Q9: How is prednisone metabolized and eliminated?
A9: Prednisone is primarily metabolized in the liver to its active form, prednisolone. [] Both prednisone and prednisolone undergo further metabolism, and the metabolites are primarily excreted in the urine. []
Q10: Does the route of administration affect prednisolone levels?
A10: Yes, intravenous administration of prednisolone phosphate results in significantly higher peak concentrations and area under the curve (AUC) compared to intravenous prednisolone phthalate or oral prednisone. []
Q11: Are there any known drug interactions that affect prednisone metabolism?
A11: Yes, rifampin, a drug known to induce hepatic enzymes, can accelerate prednisone metabolism, leading to decreased prednisone efficacy. [] In such cases, adjusting the prednisone dosage may be necessary to achieve the desired therapeutic effect. []
Q12: Can prednisone influence the pharmacokinetics of other drugs?
A12: While not directly addressed in the provided papers, prednisone's potential to induce or inhibit drug-metabolizing enzymes [] suggests a possibility of pharmacokinetic interactions with other drugs metabolized by the same enzymes.
Q13: What are the primary clinical applications of prednisone?
A13: Prednisone is used to treat various inflammatory and autoimmune diseases, including rheumatoid arthritis, [, , , ] nephrotic syndrome, [, ] giant cell arteritis, [, , , ] and idiopathic thrombocytopenic purpura (ITP). [, ]
Q14: Is prednisone effective in preventing recurrent focal glomerulosclerosis after a kidney transplant?
A14: A case study highlighted the importance of prednisone in maintaining remission of proteinuria in a patient with recurrent focal glomerulosclerosis after a kidney transplant. [] While the study suggests prednisone's potential, further research is needed to confirm its efficacy in preventing recurrence.
Q15: Are there any predictive factors for prednisone response in specific diseases?
A15: In infant acute lymphoblastic leukemia (ALL), prednisone response, defined by the degree of cytoreduction, is a strong predictor of treatment outcome. [] Patients with a good prednisone response have better event-free survival rates compared to poor responders. []
Q16: What are some known side effects associated with long-term prednisone use?
A16: Long-term prednisone use can lead to various adverse effects, including osteoporosis, [, ] cataracts, [] and an increased risk of infections. [, , ]
Q17: Does the dosage and duration of prednisone therapy affect the risk of side effects?
A17: Yes, higher doses and prolonged duration of prednisone therapy are associated with a higher risk of developing adverse effects. [, , , ]
Q18: Are there any novel drug delivery strategies being explored for prednisone?
A18: Researchers have explored biodegradable microsphere formulations using PLGA polymers for controlled and targeted delivery of prednisone. [] This approach aims to enhance drug efficacy and potentially minimize systemic side effects. [, ]
Q19: Have any biomarkers been identified to predict prednisone efficacy or monitor treatment response?
A19: A metabolomic study identified potential biomarkers in the serum of myasthenia gravis patients treated with prednisone. [] The study found changes in glycerophospholipids and arachidonic acid metabolites, suggesting their potential role as treatment response biomarkers. [, ]
Q20: Are there any environmental concerns related to prednisone?
A20: While not addressed in the provided papers, the widespread use of prednisone warrants investigation into its potential environmental impact and appropriate waste management strategies. []
Q21: Are there alternative treatments to prednisone?
A21: Yes, depending on the specific disease, several alternative treatments exist, including other immunosuppressants like cyclosporine, [, ] disease-modifying antirheumatic drugs (DMARDs) such as sulfasalazine and methotrexate, [, , ] and biological agents like tumor necrosis factor-alpha inhibitors and interleukin-1 receptor antagonists. [, , , ]
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![ethane-1,2-diol;1-isocyanato-4-[(4-isocyanatophenyl)methyl]benzene](/img/structure/B1678997.png)
![(1R,4S,5S,6S)-4-amino-2,2-dioxo-2λ6-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic acid](/img/structure/B1678998.png)