柳氮磺胺吡啶
描述
作用机制
柳氮磺胺吡啶通过多种机制发挥作用:
抗炎作用: 抑制促炎细胞因子和前列腺素的产生,减少结肠炎症.
免疫调节作用: 通过影响 T 细胞功能和细胞因子产生来调节免疫反应.
抗菌活性: 磺胺吡啶成分具有抗菌特性,有助于控制肠道菌群.
科学研究应用
生化分析
Biochemical Properties
Sulfasalazine and its metabolites, 5-aminosalicylic acid and sulfapyridine, are involved in various biochemical reactions .
Cellular Effects
Sulfasalazine has been found to have significant effects on various types of cells and cellular processes. It helps to reduce pain and swelling and lowers inflammation in the body . It limits the damage that rheumatoid arthritis causes to joints, helping to prevent disease progression .
Molecular Mechanism
One proposed mechanism is the inhibition of prostaglandins, resulting in local anti-inflammatory effects in the colon . It is also thought that Sulfasalazine and its metabolites can inhibit leukotrienes and prostaglandins by blocking the cyclo-oxygenase .
Temporal Effects in Laboratory Settings
In laboratory settings, Sulfasalazine-induced cytopenia, nephrotoxicity, and hepatotoxicity are uncommon during long-term treatment . Some guidelines recommend 3 monthly monitoring blood tests indefinitely during long-term treatment while others recommend stopping monitoring after 1 year .
Dosage Effects in Animal Models
The effects of Sulfasalazine vary with different dosages in animal models . The effects of Sulfasalazine and the analogues olsalazine, 5-aminosalicylic acid, and 4-aminosalicylic acid, in different experimental animal models have been reviewed .
Metabolic Pathways
Sulfasalazine is involved in various metabolic pathways. It is broken down by intestinal bacteria into sulfapyridine and 5-aminosalicylic acid . Sulfasalazine could improve therapy by sensitizing to cisplatin through killing CD44v9-positive cells and modifying the metabolic pathways, in particular tryptophan degradation (i.e., kynurenine pathway, serotonin pathway) and nucleic acid metabolism .
Transport and Distribution
Sulfasalazine is generally administered as enteric-coated tablets which release the drug in the small intestine . Some Sulfasalazine is absorbed in the small intestine but is limited due to transport back to the intestine by breast cancer resistance protein (ATP-binding cassette subfamily G member 2) .
Subcellular Localization
The subcellular localization of Sulfasalazine and its effects on its activity or function are not well studied. It is known that Sulfasalazine is too big to be absorbed by the small intestine, but bacteria in the colon can break the bond between 5-ASA and sulfapyridine, which frees 5-ASA to work locally in the colon to help in ulcerative colitis .
准备方法
合成路线及反应条件
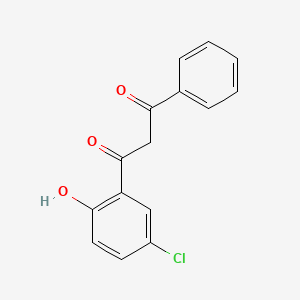
柳氮磺胺吡啶是通过一系列化学反应合成的,起始原料为磺胺吡啶和水杨酸。 该过程涉及重氮化反应,然后进行偶联反应 . 以下是合成过程的简化概述:
重氮化反应: 将磺胺吡啶溶解在含有盐酸和亚硝酸钠的水溶液中,形成重氮盐。
偶联反应: 将重氮盐与水杨酸在氢氧化钠溶液中反应,生成柳氮磺胺吡啶.
工业生产方法
在工业环境中,柳氮磺胺吡啶的合成遵循类似的步骤,但规模更大。 反应经过精心控制,以确保高产率和纯度。 最终产品通常被制成片剂或肠溶衣制剂,以提高生物利用度并减少胃肠道副作用 .
化学反应分析
反应类型
柳氮磺胺吡啶会经历多种类型的化学反应,包括:
常见的试剂和条件
水解: 由结肠中的细菌酶催化。
氧化: 通常涉及肝脏中的细胞色素 P450 酶。
还原: 可以在肠道中的厌氧条件下发生.
形成的主要产物
磺胺吡啶: 具有抗菌特性的活性代谢物。
5-氨基水杨酸: 一种在结肠局部起作用的抗炎剂.
相似化合物的比较
类似化合物
美沙拉嗪(5-氨基水杨酸): 具有抗炎特性,但缺少磺胺成分.
柳氮磺胺吡啶衍生物: 柳氮磺胺吡啶的修饰版本,具有改变的药代动力学特性.
独特性
柳氮磺胺吡啶的独特性在于它既是抗炎剂又是抗菌剂的双重作用。 它能够代谢成两种活性成分,磺胺吡啶和 5-氨基水杨酸,为治疗炎症性疾病提供了多方面的途径 .
属性
IUPAC Name |
2-hydroxy-5-[[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl]benzoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NCEXYHBECQHGNR-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N=NC3=CC(=C(C=C3)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H14N4O5S | |
| Record name | SALICYLAZOSULFAPYRIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20999 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0021256 | |
| Record name | Sulfasalazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0021256 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
398.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Salicylazosulfapyridine appears as odorless yellow or brownish-yellow to orange powder. Tasteless. (NTP, 1992) It is a sulfa drug used as an antibiotic. | |
| Record name | SALICYLAZOSULFAPYRIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20999 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
Solubility |
less than 1 mg/mL at 77 °F (NTP, 1992), Practically insoluble in water, Very slightly soluble in ethanol; practically insoluble in diethyl ether, chloroform, and benzene; soluble in aqueous solution of alkali hydroxides | |
| Record name | SALICYLAZOSULFAPYRIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20999 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Sulfasalazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3395 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Although the exact mechanism of action of sulfasalazine is not fully understood, it is thought to be mediated through the inhibition of various inflammatory molecules. Research have found that sulfasalazine and its metabolites, mesalazine and sulfapyridine, can inhibit leukotrienes and prostaglandins by blocking the cyclo-oxygenase and lipoxygenase pathway. Specific enzymes that were investigated include phospholipase A2, cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX2), and arachidonate 5-lipoxygenase., Sulfonamide combined with an anti-inflammatory drug. Sulfasalazine has little effect and salicylic acid (mesalamine) has anti-inflammatory effects. ... When administered as the combination of salicylic acid and the sulfonamide, sulfapyridine, the salicylic acid is released by colonic bacteria to produce an anti-inflammatory effect. The anti-inflammatory effect is believed to be through antiprostaglandin action, antileukotriene activity, or both., ... The purpose of the present study was to determine whether sulfasalazine therapy affected NF-kappaB activation and the expression of pro-inflammatory cytokines in patients with ulcerative colitis. ... A total of 38 patients with moderate ulcerative colitis were investigated. Twenty-one patients received sulfasalazine. Seventeen patients did not receive any medication. Biopsy specimens were obtained from inflamed mucosa and analyzed for NF-kappaB DNA binding activity, NF-kappaBp65/IkappaBalpha protein expression and the levels of pro-inflammatory cytokine mRNA using electrophoretic mobility shift assay, western blot analysis, immunohistochemical staining and reverse transcription-polymerase chain reaction (RT-PCR) analysis, respectively. Increased activation of NF-kappaB and high levels of the expression of interleukin (IL)-1beta mRNA and IL-8 mRNA were detected in biopsy specimens from patients with ulcerative colitis. Therapeutic administration of sulfasalazine effectively downregulated the activation of NF-kappaB and the expression of IL-1beta mRNA and IL-8 mRNA while IkappaBalpha levels were stable. /The authors concluded that/ the therapeutic benefits for ulcerative colitis of sulfasalazine might at least in part be attributed to its ability to inhibit NF-kappaB activation, resulting in the downregulation of pro-inflammatory cytokine mRNA expression. | |
| Record name | Sulfasalazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00795 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Sulfasalazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3395 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Minute, brownish-yellow crystals, Light brownish yellow to bright yellow fine powder | |
CAS No. |
599-79-1 | |
| Record name | SALICYLAZOSULFAPYRIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20999 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Sulfasalazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=599-79-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Sulfasalazine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000599791 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Sulfasalazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00795 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | sulfasalazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757330 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | sulfasalazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=667219 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | sulfasalazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=203730 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Sulfasalazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0021256 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Salazosulfapyridine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.009.069 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | SULFASALAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3XC8GUZ6CB | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Sulfasalazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3395 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
464 to 473 °F (decomposes) (NTP, 1992), 220 °C (decomposes) | |
| Record name | SALICYLAZOSULFAPYRIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20999 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Sulfasalazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00795 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Sulfasalazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3395 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![3-amino-6,6-dimethyl-2-phenyl-8H-pyrano[4,5-e]pyridazine-4-carbonitrile](/img/structure/B1682630.png)