Patent
US08202910B2
Procedure details


Antimicrobial drugs isoniazid, rifampicin, streptomycin, and ethambutol were purchased from Sigma-Aldrich. St. Louis, Mo. Stock solutions of isoniazid, streptomycin, and ethambutol were prepared in distilled and deionized water at 10 mg/mL, sterilized by filtration, and stored frozen at −80° C. Stock solutions of rifampicin, at 1 or 10 mg/mL, were prepared in methanol and stored at −80° C. Pyrazinamide was purchased as the drug reconstituting kit from Becton Dickinson, Cockeysville, Md., and a stock solution was prepared following instructions by the manufacturer. Stock solutions of SQ109 were prepared in methanol at 1 mg/mL and stored at 80° C.



Name
streptomycin
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Four




Name
Identifiers


|
REACTION_CXSMILES
|
[CH:1]1[C:6]([C:7]([NH:9][NH2:10])=[O:8])=[CH:5][CH:4]=[N:3][CH:2]=1.CC1C2O[C@:19]3(C)OC=C[C@H](OC)[C@@H](C)[C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)C=CC=[C:36](C)[C:37]([NH:39][C:40]4[C:43](/[CH:46]=N/N5CCN(C)CC5)=[C:44](O)[C:15]([C:16]=2[C:20]3=O)=[C:14]([C:41]=4O)C=1O)=O.[CH3:70][C@@H:71]1[O:75][C@@H:74]([O:76][C@H:77]2[C@H:82]([OH:83])[C@@H:81]([OH:84])[C@H:80]([NH:85][C:86]([NH2:88])=[NH:87])[C@@H:79]([OH:89])[C@@H:78]2[NH:90][C:91]([NH2:93])=[NH:92])[C@H:73]([O:94][C@@H:95]2[O:100][C@@H:99]([CH2:101][OH:102])[C@H:98]([OH:103])[C@@H:97]([OH:104])[C@@H:96]2[NH:105][CH3:106])[C@@:72]1([OH:109])[CH:107]=[O:108].[CH3:110][CH2:111][C@H:112]([NH:115][CH2:116][CH2:117][NH:118][C@H:119]([CH2:122][OH:123])[CH2:120][CH3:121])[CH2:113][OH:114].N1C=CN=CC=1C(N)=O>CO>[CH:1]1[C:6]([C:7]([NH:9][NH2:10])=[O:8])=[CH:5][CH:4]=[N:3][CH:2]=1.[CH3:70][C@@H:71]1[O:75][C@@H:74]([O:76][C@H:77]2[C@H:82]([OH:83])[C@@H:81]([OH:84])[C@H:80]([NH:85][C:86]([NH2:88])=[NH:87])[C@@H:79]([OH:89])[C@@H:78]2[NH:90][C:91]([NH2:93])=[NH:92])[C@H:73]([O:94][C@@H:95]2[O:100][C@@H:99]([CH2:101][OH:102])[C@H:98]([OH:103])[C@@H:97]([OH:104])[C@@H:96]2[NH:105][CH3:106])[C@@:72]1([OH:109])[CH:107]=[O:108].[CH3:121][CH2:120][C@H:119]([NH:118][CH2:117][CH2:116][NH:115][C@H:112]([CH2:113][OH:114])[CH2:111][CH3:110])[CH2:122][OH:123].[CH3:73][C:72]([CH3:107])=[CH:71][CH2:4][CH2:5]/[C:6](/[CH3:7])=[CH:1]/[CH2:2][NH:3][CH2:36][CH2:37][NH:39][CH:40]1[CH:41]2[CH2:14][CH:15]3[CH2:16][CH:20]([CH2:19]2)[CH2:46][CH:43]1[CH2:44]3
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
CO
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
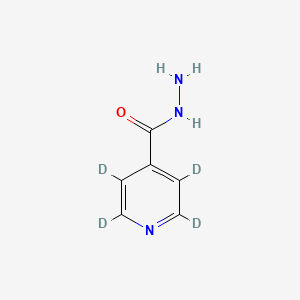
C1=CN=CC=C1C(=O)NN
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C2=C3C4=C1O[C@@](C4=O)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]([C@H](/C=C/C=C(\C(=O)NC(=C2O)C(=C3O)/C=N/N5CCN(CC5)C)/C)C)O)C)O)C)OC(=O)C)C)OC)C)O
|
Step Four
|
Name
|
streptomycin
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC[C@@H](CO)NCCN[C@@H](CC)CO
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
N1=C(C=NC=C1)C(=O)N
|
Conditions


Temperature
|
Control Type
|
UNSPECIFIED
|
|
Setpoint
|
-80 °C
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


DISTILLATION
|
Type
|
DISTILLATION
|
|
Details
|
in distilled
|
FILTRATION
|
Type
|
FILTRATION
|
|
Details
|
deionized water at 10 mg/mL, sterilized by filtration
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Stock solutions of rifampicin, at 1 or 10 mg/mL, were prepared in methanol
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
stored at −80° C
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
, and a stock solution was prepared
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
stored at 80° C.
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C1=CN=CC=C1C(=O)NN
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC[C@@H](CO)NCCN[C@@H](CC)CO
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC(=CCC/C(=C/CNCCNC1C2CC3CC(C2)CC1C3)/C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US08202910B2
Procedure details


Antimicrobial drugs isoniazid, rifampicin, streptomycin, and ethambutol were purchased from Sigma-Aldrich. St. Louis, Mo. Stock solutions of isoniazid, streptomycin, and ethambutol were prepared in distilled and deionized water at 10 mg/mL, sterilized by filtration, and stored frozen at −80° C. Stock solutions of rifampicin, at 1 or 10 mg/mL, were prepared in methanol and stored at −80° C. Pyrazinamide was purchased as the drug reconstituting kit from Becton Dickinson, Cockeysville, Md., and a stock solution was prepared following instructions by the manufacturer. Stock solutions of SQ109 were prepared in methanol at 1 mg/mL and stored at 80° C.



Name
streptomycin
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Four




Name
Identifiers


|
REACTION_CXSMILES
|
[CH:1]1[C:6]([C:7]([NH:9][NH2:10])=[O:8])=[CH:5][CH:4]=[N:3][CH:2]=1.CC1C2O[C@:19]3(C)OC=C[C@H](OC)[C@@H](C)[C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)C=CC=[C:36](C)[C:37]([NH:39][C:40]4[C:43](/[CH:46]=N/N5CCN(C)CC5)=[C:44](O)[C:15]([C:16]=2[C:20]3=O)=[C:14]([C:41]=4O)C=1O)=O.[CH3:70][C@@H:71]1[O:75][C@@H:74]([O:76][C@H:77]2[C@H:82]([OH:83])[C@@H:81]([OH:84])[C@H:80]([NH:85][C:86]([NH2:88])=[NH:87])[C@@H:79]([OH:89])[C@@H:78]2[NH:90][C:91]([NH2:93])=[NH:92])[C@H:73]([O:94][C@@H:95]2[O:100][C@@H:99]([CH2:101][OH:102])[C@H:98]([OH:103])[C@@H:97]([OH:104])[C@@H:96]2[NH:105][CH3:106])[C@@:72]1([OH:109])[CH:107]=[O:108].[CH3:110][CH2:111][C@H:112]([NH:115][CH2:116][CH2:117][NH:118][C@H:119]([CH2:122][OH:123])[CH2:120][CH3:121])[CH2:113][OH:114].N1C=CN=CC=1C(N)=O>CO>[CH:1]1[C:6]([C:7]([NH:9][NH2:10])=[O:8])=[CH:5][CH:4]=[N:3][CH:2]=1.[CH3:70][C@@H:71]1[O:75][C@@H:74]([O:76][C@H:77]2[C@H:82]([OH:83])[C@@H:81]([OH:84])[C@H:80]([NH:85][C:86]([NH2:88])=[NH:87])[C@@H:79]([OH:89])[C@@H:78]2[NH:90][C:91]([NH2:93])=[NH:92])[C@H:73]([O:94][C@@H:95]2[O:100][C@@H:99]([CH2:101][OH:102])[C@H:98]([OH:103])[C@@H:97]([OH:104])[C@@H:96]2[NH:105][CH3:106])[C@@:72]1([OH:109])[CH:107]=[O:108].[CH3:121][CH2:120][C@H:119]([NH:118][CH2:117][CH2:116][NH:115][C@H:112]([CH2:113][OH:114])[CH2:111][CH3:110])[CH2:122][OH:123].[CH3:73][C:72]([CH3:107])=[CH:71][CH2:4][CH2:5]/[C:6](/[CH3:7])=[CH:1]/[CH2:2][NH:3][CH2:36][CH2:37][NH:39][CH:40]1[CH:41]2[CH2:14][CH:15]3[CH2:16][CH:20]([CH2:19]2)[CH2:46][CH:43]1[CH2:44]3
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
CO
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C1=CN=CC=C1C(=O)NN
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C2=C3C4=C1O[C@@](C4=O)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]([C@H](/C=C/C=C(\C(=O)NC(=C2O)C(=C3O)/C=N/N5CCN(CC5)C)/C)C)O)C)O)C)OC(=O)C)C)OC)C)O
|
Step Four
|
Name
|
streptomycin
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC[C@@H](CO)NCCN[C@@H](CC)CO
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
N1=C(C=NC=C1)C(=O)N
|
Conditions


Temperature
|
Control Type
|
UNSPECIFIED
|
|
Setpoint
|
-80 °C
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


DISTILLATION
|
Type
|
DISTILLATION
|
|
Details
|
in distilled
|
FILTRATION
|
Type
|
FILTRATION
|
|
Details
|
deionized water at 10 mg/mL, sterilized by filtration
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Stock solutions of rifampicin, at 1 or 10 mg/mL, were prepared in methanol
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
stored at −80° C
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
, and a stock solution was prepared
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
stored at 80° C.
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C1=CN=CC=C1C(=O)NN
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC[C@@H](CO)NCCN[C@@H](CC)CO
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC(=CCC/C(=C/CNCCNC1C2CC3CC(C2)CC1C3)/C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |