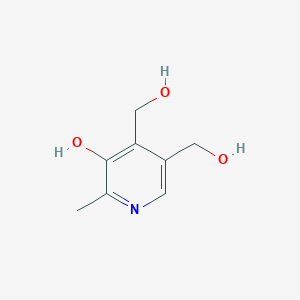
Pyridoxine
Overview
Description
Pyridoxine, commonly known as vitamin B6, is a water-soluble vitamin that plays a crucial role in various biological functions. It is found naturally in many foods and is also available as a dietary supplement. This compound is essential for the metabolism of amino acids, carbohydrates, and lipids, and it supports brain health, immune function, and the synthesis of neurotransmitters .
Preparation Methods
Synthetic Routes and Reaction Conditions: Pyridoxine can be synthesized through various methods. One common approach involves the condensation of cyanoacetamide with 1,3-dicarbonyl compounds. Another method uses the condensation of 1,3-oxazole derivatives with dienophiles, followed by catalytic hydrogenation . These methods are characterized by high yields and mild reaction conditions.
Industrial Production Methods: The industrial production of this compound typically involves the “oxazole” method, which is a two-stage process. The first stage involves the diene condensation to form intermediate compounds, which are then converted into this compound through catalytic hydrogenation . This method is widely used due to its efficiency and cost-effectiveness.
Chemical Reactions Analysis
Types of Reactions: Pyridoxine undergoes various chemical reactions, including oxidation, reduction, and substitution. It can be selectively oxidized to form pyridoxal or pyridoxal hydrochloride using a catalytic oxidation system .
Common Reagents and Conditions: Common reagents used in the oxidation of this compound include oxygen sources, catalysts, inorganic salts, and amine ligands. The reactions are typically carried out in water as the solvent under mild conditions .
Major Products: The major products formed from the oxidation of this compound are pyridoxal and pyridoxal hydrochloride, which are key intermediates in the synthesis of pyridoxal 5’-phosphate, the active coenzyme form of vitamin B6 .
Scientific Research Applications
Pyridoxine has a wide range of scientific research applications:
Mechanism of Action
Pyridoxine is converted to pyridoxal 5’-phosphate in the body, which acts as a coenzyme in various biochemical reactions. It is involved in the metabolism of amino acids, glycogen, and lipids, and it aids in the synthesis of neurotransmitters such as serotonin, dopamine, norepinephrine, and gamma-aminobutyric acid (GABA) . Pyridoxal 5’-phosphate also plays a role in the synthesis of hemoglobin and sphingolipids .
Comparison with Similar Compounds
- Pyridoxal
- Pyridoxamine
- Pyridoxine 5’-phosphate
- Pyridoxal 5’-phosphate
- Pyridoxamine 5’-phosphate
This compound is unique in its stability and is the most commonly used form in dietary supplements .
Properties
IUPAC Name |
4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H11NO3/c1-5-8(12)7(4-11)6(3-10)2-9-5/h2,10-12H,3-4H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LXNHXLLTXMVWPM-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NC=C(C(=C1O)CO)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H11NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
58-56-0 (hydrochloride) | |
| Record name | Pyridoxine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000065236 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4023541 | |
| Record name | Pyridoxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4023541 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
169.18 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
White powder; [Alfa Aesar MSDS], Solid | |
| Record name | Pyridoxine | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/17041 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
79 mg/mL | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
0.00000028 [mmHg] | |
| Record name | Pyridoxine | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/17041 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
Mechanism of Action |
Vitamin B6 is the collective term for a group of three related compounds, pyridoxine (PN), pyridoxal (PL) and pyridoxamine (PM), and their phosphorylated derivatives, pyridoxine 5'-phosphate (PNP), pyridoxal 5'-phosphate (PLP) and pyridoxamine 5'-phosphate (PMP). Although all six of these compounds should technically be referred to as vitamin B6, the term vitamin B6 is commonly used interchangeably with just one of them, pyridoxine. Vitamin B6, principally in its biologically active coenzyme form pyridoxal 5'-phosphate, is involved in a wide range of biochemical reactions, including the metabolism of amino acids and glycogen, the synthesis of nucleic acids, hemogloblin, sphingomyelin and other sphingolipids, and the synthesis of the neurotransmitters serotonin, dopamine, norepinephrine and gamma-aminobutyric acid (GABA). | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
65-23-6 | |
| Record name | Pyridoxine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=65-23-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pyridoxine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000065236 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | pyridoxine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759148 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 3,4-Pyridinedimethanol, 5-hydroxy-6-methyl- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Pyridoxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4023541 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Pyridoxine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.548 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PYRIDOXINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/KV2JZ1BI6Z | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
159-162 °C, 159 - 162 °C | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details











Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
ANone: Pyridoxine is a precursor to pyridoxal 5'-phosphate (PLP), the active form of vitamin B6. PLP functions as a crucial coenzyme for over 100 enzymes involved in various metabolic pathways. [] This includes the metabolism of amino acids, carbohydrates, lipids, neurotransmitters, and heme. [, , ]
ANone: this compound, through its conversion to PLP, plays a vital role in the biosynthesis of several neurotransmitters, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and histamine. [, , ] It participates in decarboxylation and transamination reactions essential for their production.
ANone: this compound deficiency can disrupt the synthesis of neurotransmitters. For instance, inadequate this compound levels can lead to reduced GABA synthesis, potentially contributing to seizures. [, ] This highlights the critical role of this compound in maintaining normal neurological function.
ANone: The molecular formula of this compound is C8H11NO3, and its molecular weight is 169.18 g/mol.
ANone: While the provided research papers focus primarily on the biological effects of this compound, several analytical techniques have been employed. UV spectrophotometry is one such method used to quantify this compound in pharmaceutical formulations, utilizing its absorption characteristics. [, , ]
ANone: this compound, when incorporated into soybean lecithin-based extenders for goat semen, demonstrated beneficial effects on sperm quality after the freeze-thawing process. [] This suggests the compatibility and potential stabilizing properties of this compound in specific biological formulations.
ANone: The study on 4'-deoxythis compound provides insights into the structure-activity relationship of this compound. This compound, a this compound analog, demonstrated the ability to both inhibit and stimulate the growth of an Escherichia coli mutant, depending on the concentration of pyridoxal. [] This suggests that even subtle modifications to the this compound structure can significantly alter its biological effects.
ANone: While specific formulation strategies were not discussed in the provided abstracts, research involving this compound often focuses on its administration and bioavailability. For instance, in the treatment of this compound-dependent epilepsy, the timing of this compound supplementation, including antenatal administration, has been explored to optimize its therapeutic efficacy. [] This highlights the ongoing efforts to improve the delivery and effectiveness of this compound in clinical settings.
ANone: Information pertaining to SHE regulations was not covered in the provided research.
ANone: Research suggests that chronic levodopa administration, commonly used in Parkinson's disease, might influence this compound metabolism. [] Patients on chronic levodopa treatment exhibited higher plasma and erythrocyte PLP concentrations after receiving intravenous this compound compared to levodopa-naive controls. [] This finding suggests an adaptive alteration in this compound metabolism induced by levodopa, highlighting the complex interplay between medications and nutrient metabolism.
ANone: Studies using mouse colonic epithelial cells and human colonic apical membrane vesicles revealed that this compound uptake is a carrier-mediated process, suggesting the existence of specific transporters for this compound absorption in the colon. [] Similarly, research on pancreatic acinar cells demonstrated a regulatable and specific carrier-mediated mechanism for this compound uptake, indicating that different cell types may possess unique mechanisms for this compound transport and utilization. []
ANone: Research in rats indicates that this compound might offer protection against the toxic effects of linezolid, an antibiotic. [] Co-administration of this compound with linezolid attenuated hematological toxicity, hepatotoxicity, and oxidative stress markers in rats, suggesting a potential role for this compound in mitigating drug-induced adverse effects. []
ANone: While the exact mechanisms underlying PDE are still being elucidated, research has established a strong link between mutations in the ALDH7A1 gene, responsible for encoding alpha-aminoadipic semialdehyde (AASA) dehydrogenase, and the development of PDE. [, ] These mutations can lead to a deficiency in AASA dehydrogenase activity, resulting in the accumulation of AASA and the development of seizures. [, ] Supplementation with this compound can alleviate seizures in affected individuals, although the specific mechanisms underlying its therapeutic benefits are not fully understood. []
ANone: Research on Jian carp suggests that dietary this compound supplementation can enhance disease resistance and immune responses in fish. [] Fish fed diets containing this compound exhibited higher survival rates after bacterial challenge, along with improvements in various immune parameters. [] These findings highlight the potential of this compound as a dietary supplement for promoting fish health and resilience in aquaculture settings.
ANone: The provided abstracts do not contain information about resistance or cross-resistance to this compound.
ANone: The provided abstracts do not contain information about toxicity or long-term effects of this compound.
ANone: While specific drug delivery strategies for this compound were not discussed in detail, research highlights the importance of its bioavailability and transport. For example, studies using isolated rat liver cells investigated the uptake and metabolism of this compound glucosides. [] This research suggests that the form in which this compound is present in food can affect its absorption and utilization by the body.
ANone: Urinary α-aminoadipic semialdehyde (aAASA) levels have emerged as a potential biomarker for PDE. [] Elevated aAASA levels in urine can indicate a deficiency in AASA dehydrogenase activity, which is the underlying metabolic defect in PDE. [] Monitoring aAASA levels could help clinicians assess the effectiveness of this compound treatment and adjust dosages as needed.
ANone: Researchers utilize various analytical techniques to study this compound. High-performance liquid chromatography (HPLC) coupled with UV detection is one method for quantifying this compound in multivitamin preparations. [, ] This technique allows for the separation and measurement of this compound and other vitamins in complex mixtures.
ANone: Researchers prioritize the validation of analytical methods used in this compound research. A study validating an HPLC method for simultaneous analysis of metamizole, thiamine, and this compound in tablets highlights the importance of accuracy, precision, and specificity in analytical measurements. [] By adhering to strict validation procedures, researchers ensure the quality and reliability of their data, which is crucial for making accurate interpretations and drawing meaningful conclusions.
ANone: The provided research abstracts did not focus on these aspects related to this compound.
ANone: The recognition of this compound as an essential nutrient and the subsequent characterization of this compound deficiency syndromes represent significant milestones. [, ] Early research established the link between this compound deficiency and various conditions, including dermatitis, anemia, and neurological disorders. [, ]
ANone: this compound research extends beyond the realm of nutrition science and demonstrates significant overlap with other disciplines. For instance, the investigation of this compound-dependent epilepsy involves collaboration between geneticists, neurologists, and biochemists to unravel the complex interplay between genetic mutations, metabolic pathways, and clinical manifestations. [, ]
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.

![Pentapotassium;2-[6-[bis(carboxylatomethyl)amino]-5-[2-[6-[bis(carboxylatomethyl)amino]-2,3-difluorophenoxy]ethoxy]-1-benzofuran-2-yl]-1,3-oxazole-5-carboxylate](/img/structure/B162722.png)