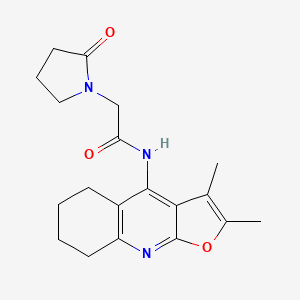
Coluracetam
Overview
Description
Coluracetam, also known by its chemical name N-(2,3-dimethyl-5,6,7,8-tetrahydrofuro[2,3-b]quinolin-4-yl)-2-(2-oxo-1-pyrrolidinyl)acetamide, is a member of the racetam family of nootropic compounds. Initially developed by Mitsubishi Tanabe Pharma Corporation for the treatment of Alzheimer’s disease, it has since been explored for its potential benefits in enhancing cognitive function, memory, and mood .
Mechanism of Action
Target of Action
The primary target of Coluracetam is the high-affinity choline uptake (HACU) in neurons . Choline is a precursor to the neurotransmitter acetylcholine, which plays a crucial role in cognitive processes such as memory formation, learning, and attention .
Mode of Action
This compound interacts with its target, the high-affinity choline transporter (HACU), which is responsible for transporting choline across the cell membrane and into neurons . By enhancing the efficiency of the choline transport system, this compound promotes the synthesis and release of acetylcholine, ultimately leading to improved cognitive function .
Biochemical Pathways
This compound affects the cholinergic system, specifically the pathway of acetylcholine synthesis . By enhancing high-affinity choline uptake, this compound increases the levels of acetylcholine, a crucial neurotransmitter associated with memory and cognition . This increase can help with memory formation and retention, making the brain work more efficiently and supporting various mental tasks .
Pharmacokinetics
This compound appears to have very rapid kinetics, with a peak in blood at around 30 minutes and on the decline within 3 hours . Due to this, supplementation may be time-dependent in relation to activity . It is fat-soluble, so it needs fats for optimal absorption . It’s usually taken sublingually (under the tongue) or orally . This method enhances its bioavailability .
Result of Action
The molecular and cellular effects of this compound’s action are primarily related to its enhancement of acetylcholine synthesis. By increasing the levels of acetylcholine, this compound improves cognitive functions such as memory formation, learning, and attention . It has been shown to improve learning impairment on a single oral dose given to rats which have been exposed to cholinergic neurotoxins .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the presence of fats in the environment (i.e., the user’s diet) can affect the absorption of this fat-soluble compound . Furthermore, the timing of supplementation in relation to activity can influence the compound’s effects due to its rapid kinetics .
Biochemical Analysis
Biochemical Properties
The primary mechanism of action for coluracetam is its ability to increase the uptake of choline into neurons . Choline is a precursor to the neurotransmitter acetylcholine, which plays a crucial role in cognitive processes such as memory formation, learning, and attention . This compound achieves this by interacting with the high-affinity choline transporter (HACU), which is responsible for transporting choline across the cell membrane and into neurons . By enhancing the efficiency of the choline transport system, this compound promotes the synthesis and release of acetylcholine .
Cellular Effects
This compound works mainly by increasing the levels of acetylcholine, a crucial neurotransmitter, and modulating choline uptake in the brain . Its unique mechanisms make it interesting for cognitive enhancement and potential therapeutic uses . Enhancement of Acetylcholine Synthesis: this compound boosts acetylcholine synthesis . Acetylcholine is vital for learning and memory . The brain uses choline to produce acetylcholine . By enhancing this process, this compound improves cognitive functions .
Molecular Mechanism
This compound enhances high-affinity choline uptake (HACU), which is the rate-limiting step of acetylcholine (ACh) synthesis . Studies have shown this compound to improve learning impairment on a single oral dose given to rats which have been exposed to cholinergic neurotoxins . Subsequent studies have shown that it may induce long-lasting procognitive effects in cholinergic neurotoxin-treated rats by changing the choline transporter regulation system .
Dosage Effects in Animal Models
Most of the research on this compound was done on animals rather than humans, so there are no universally accepted dosing guidelines . In a 2010 animal study, this compound improved artificially-induced memory deficits without producing any significant side effects .
Transport and Distribution
This compound is fat-soluble, so it needs fats for optimal absorption . It’s usually taken sublingually (under the tongue) or orally . This method enhances its bioavailability .
Subcellular Localization
Given its mechanism of action, it is likely that this compound interacts with neurons in the brain where it enhances the uptake of choline, a precursor to the neurotransmitter acetylcholine .
Side effects and long-term safety remain areas requiring more comprehensive research .
Preparation Methods
Synthetic Routes and Reaction Conditions: Coluracetam is synthesized through a multi-step process involving the reaction of 2,3-dimethyl-5,6,7,8-tetrahydrofuro[2,3-b]quinoline with 2-oxo-1-pyrrolidineacetamide. The key steps include:
Formation of the quinoline core: This involves cyclization reactions under controlled conditions.
Attachment of the pyrrolidinyl group: This step typically requires the use of coupling reagents and catalysts to ensure high yield and purity.
Industrial Production Methods: Industrial production of this compound follows similar synthetic routes but on a larger scale. The process is optimized for efficiency, cost-effectiveness, and adherence to regulatory standards. Key considerations include:
Reaction scalability: Ensuring that the reactions can be scaled up without loss of yield or purity.
Purification: Employing techniques such as crystallization and chromatography to achieve the desired purity levels.
Chemical Reactions Analysis
Types of Reactions: Coluracetam undergoes various chemical reactions, including:
Oxidation: This can occur under specific conditions, leading to the formation of oxidized derivatives.
Reduction: Reduction reactions can modify the functional groups, potentially altering the compound’s activity.
Substitution: Substitution reactions can introduce different functional groups, which may enhance or diminish its nootropic properties.
Common Reagents and Conditions:
Oxidizing agents: Such as potassium permanganate or hydrogen peroxide.
Reducing agents: Including sodium borohydride or lithium aluminum hydride.
Catalysts: Often used in substitution reactions to increase reaction rates and yields.
Major Products: The primary products of these reactions are modified forms of this compound, which may have different pharmacological properties. These derivatives are often studied to understand structure-activity relationships.
Scientific Research Applications
Coluracetam has been investigated for various scientific research applications:
Chemistry: Studied for its unique chemical structure and potential to form novel derivatives.
Biology: Explored for its effects on neurotransmitter systems, particularly acetylcholine.
Medicine: Investigated for its potential to treat cognitive disorders, depression, and anxiety.
Industry: Used in the development of nootropic supplements aimed at enhancing cognitive function and mood.
Comparison with Similar Compounds
Piracetam: The first racetam nootropic, known for its cognitive-enhancing effects.
Aniracetam: Another racetam with anxiolytic and cognitive benefits.
Oxiracetam: Known for its stimulating effects and cognitive enhancement.
Uniqueness of Coluracetam: this compound is unique due to its specific mechanism of enhancing high-affinity choline uptake, which is not observed in other racetams. This distinct action makes it particularly effective in improving memory and learning in conditions where cholinergic function is compromised .
Properties
IUPAC Name |
N-(2,3-dimethyl-5,6,7,8-tetrahydrofuro[2,3-b]quinolin-4-yl)-2-(2-oxopyrrolidin-1-yl)acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H23N3O3/c1-11-12(2)25-19-17(11)18(13-6-3-4-7-14(13)20-19)21-15(23)10-22-9-5-8-16(22)24/h3-10H2,1-2H3,(H,20,21,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PSPGQHXMUKWNDI-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(OC2=NC3=C(CCCC3)C(=C12)NC(=O)CN4CCCC4=O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H23N3O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60159386 | |
| Record name | Coluracetam | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60159386 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
341.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
135463-81-9 | |
| Record name | Coluracetam | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=135463-81-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Coluracetam [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0135463819 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Coluracetam | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60159386 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | COLURACETAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/V6FL6O5GR7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.