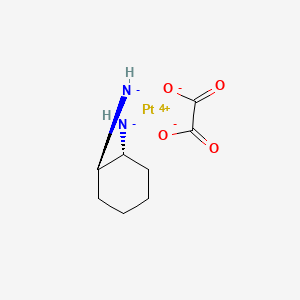
Oxaliplatin
Overview
Description
Oxaliplatin is a platinum-based chemotherapy drug used primarily in the treatment of colorectal cancer. It belongs to the class of platinum-containing antineoplastic agents and is known for its ability to form cross-links with DNA, thereby inhibiting DNA replication and transcription. This compound has shown efficacy in treating various types of cancer, including colorectal, ovarian, and pancreatic cancers .
Mechanism of Action
Target of Action
Oxaliplatin, a platinum-based chemotherapy drug, primarily targets DNA within cancer cells . The platinum compound in this compound binds to DNA, forming cross-links that inhibit DNA replication and transcription .
Mode of Action
This compound undergoes nonenzymatic conversion in physiological solutions to active derivatives via displacement of the labile oxalate ligand . These active derivatives covalently bind with macromolecules, forming both inter and intrastrand Pt-DNA crosslinks . The structure of this compound makes the binding of mismatch repair protein to DNA harder compared to cisplatin or carboplatin’s adducts, resulting in greater cytotoxic effects .
Biochemical Pathways
The main mechanism of action of this compound is believed to be the induction of cancer cell apoptosis as a response to its covalent binding to DNA . This stops or slows the growth of cancer cells and other rapidly growing cells and causes them to die . This compound also modulates STAT signaling and enhances the effector immune response through modulation of programmed death receptor 1-ligand and mannose-6-phosphate receptor expression .
Pharmacokinetics
The pharmacokinetics of unbound platinum in plasma ultrafiltrate after this compound administration is triphasic, characterized by a short initial distribution phase and a long terminal elimination phase . No accumulation was observed in plasma ultrafiltrate after 130 mg/m² every 3 weeks or 85 mg/m² every 2 weeks . Urinary excretion (53.8 ± 9.1%) was the predominant route of platinum elimination, with fecal excretion accounting for only 2.1 ± 1.9% of the administered dose 5 days post-administration .
Result of Action
The result of this compound’s action is primarily the induction of apoptosis in cancer cells . This is achieved through the formation of DNA crosslinks that inhibit DNA replication and transcription, leading to cell death . This compound has shown clinical efficacy against many solid tumors, including colorectal cancer .
Action Environment
This compound is typically administered in combination with fluorouracil and leucovorin, known as the FOLFOX regimen, for the treatment of colorectal cancer . The environment in which this compound is administered can influence its action, efficacy, and stability. For instance, renal function can impact the clearance of this compound, with lower clearance observed in patients with moderate renal impairment . No marked increase in drug toxicity was reported in these patients .
Biochemical Analysis
Biochemical Properties
Oxaliplatin interacts with various enzymes, proteins, and other biomolecules. Its mechanism of action primarily involves DNA damage through DNA crosslinking, particularly intrastrand and interstrand crosslinking . The structure of this compound allows its adducts to bind mismatch repair proteins to DNA more difficultly compared to cisplatin or carboplatin’s adducts, resulting in greater cytotoxic effects .
Cellular Effects
This compound has a cytotoxic effect in a broad range of cell lines, including colon, ovarian, and lung cancer . It damages the DNA of cells and stops it from being copied. This stops or slows the growth of cancer cells and other rapidly growing cells and causes them to die .
Molecular Mechanism
The molecular mechanism of this compound involves the formation of platinum-DNA adducts. The retention of the bulky DACH ring by activated this compound results in the formation of these adducts, which appear to be more effective at blocking DNA replication and are more cytotoxic than adducts formed from cisplatin .
Temporal Effects in Laboratory Settings
The pharmacokinetics of unbound platinum in plasma ultrafiltrate after this compound administration is triphasic, characterized by a short initial distribution phase and a long terminal elimination phase . No accumulation was observed in plasma ultrafiltrate after 130 mg/m^2 every 3 weeks or 85 mg/m^2 every 2 weeks .
Dosage Effects in Animal Models
In animal models, the effects of this compound vary with different dosages. For example, in mice, PKM2 silencing induced resistance in HT29 and SW480 cells and sensitivity in HCT116 cells . The highest recommended dose of this compound in patients is 110 mg/m^2 (2.97 mg/kg) .
Metabolic Pathways
This compound is involved in various metabolic pathways. Accumulated doses of this compound led to significant changes in gene expression related to energy-related metabolic pathways including fatty acid oxidation, amino acid metabolism, glycolysis, electron transport chain, and NAD synthesis pathway .
Transport and Distribution
This compound is transported and distributed within cells and tissues. The copper transporter 1 seems to be of particular importance for cisplatin uptake in tumor cells, while the organic cation transporter (OCT) 2, due to its specific organ distribution, may play a major role in the development of undesired cisplatin side effects .
Subcellular Localization
This compound undergoes nuclear translocation in response to this compound in HCT116 and HT29 cells but not in OXA-resistant HTOXAR3 cells . This suggests that the subcellular localization of this compound may play a role in its effectiveness and resistance.
Preparation Methods
Synthetic Routes and Reaction Conditions: Oxaliplatin is synthesized through a multi-step process involving the reaction of platinum compounds with specific ligands. The primary synthetic route involves the reaction of cis-diiodo(trans-1,2-diaminocyclohexane)platinum(II) with oxalic acid. The reaction conditions typically include a controlled temperature and pH to ensure the formation of the desired product .
Industrial Production Methods: In industrial settings, this compound is produced using large-scale reactors where the reaction conditions are meticulously controlled to achieve high yields and purity. The process involves the use of high-purity starting materials and solvents, and the final product is subjected to rigorous quality control measures to ensure its efficacy and safety .
Chemical Reactions Analysis
Types of Reactions: Oxaliplatin undergoes several types of chemical reactions, including substitution, oxidation, and reduction. The most notable reaction is its ability to form covalent bonds with DNA, leading to the formation of intrastrand and interstrand cross-links .
Common Reagents and Conditions: The reactions involving this compound often require specific reagents such as chloride ions, water, and various organic solvents. The conditions typically include controlled temperatures and pH levels to facilitate the desired chemical transformations .
Major Products Formed: The primary products formed from the reactions of this compound are DNA adducts, which are responsible for its cytotoxic effects. These adducts prevent DNA replication and transcription, leading to cell death .
Scientific Research Applications
Oxaliplatin has a wide range of applications in scientific research, particularly in the fields of chemistry, biology, medicine, and industry.
Chemistry: In chemistry, this compound is used as a model compound to study the interactions of platinum-based drugs with DNA and other biomolecules. Researchers investigate its reactivity and binding properties to develop new and more effective platinum-based drugs .
Biology: In biological research, this compound is used to study the mechanisms of drug resistance in cancer cells. Scientists explore how cancer cells develop resistance to platinum-based drugs and seek ways to overcome this resistance .
Medicine: In medicine, this compound is a cornerstone in the treatment of colorectal cancer. It is often used in combination with other chemotherapeutic agents such as fluorouracil and leucovorin in regimens like FOLFOX. Clinical trials continue to explore its efficacy in treating other types of cancer .
Industry: In the pharmaceutical industry, this compound is produced and formulated into injectable solutions for clinical use. Its production involves stringent quality control measures to ensure its safety and efficacy for patients .
Comparison with Similar Compounds
Cisplatin: Known for its efficacy in treating testicular, ovarian, and bladder cancers. .
Carboplatin: Used in the treatment of ovarian and lung cancers. .
Uniqueness of Oxaliplatin: this compound is unique in its ability to form more stable DNA adducts, leading to greater cytotoxicity in cancer cells. It also has a different side effect profile, with a lower incidence of nephrotoxicity and ototoxicity but a higher incidence of peripheral neuropathy .
Properties
| Oxaliplatin undergoes nonenzymatic conversion in physiologic solutions to active derivatives via displacement of the labile oxalate ligand. Several transient reactive species are formed, including monoaquo and diaquo DACH platinum, which covalently bind with macromolecules. Both inter and intrastrand Pt-DNA crosslinks are formed. Crosslinks are formed between the N7 positions of two adjacent guanines (GG), adjacent adenine-guanines (AG), and guanines separated by an intervening nucleotide (GNG). These crosslinks inhibit DNA replication and transcription. Cytotoxicity is cell-cycle nonspecific. | |
CAS No. |
61825-94-3 |
Molecular Formula |
C8H14N2O4Pt |
Molecular Weight |
397.29 g/mol |
IUPAC Name |
[(1R,2R)-2-azanidylcyclohexyl]azanide;oxalic acid;platinum(2+) |
InChI |
InChI=1S/C6H12N2.C2H2O4.Pt/c7-5-3-1-2-4-6(5)8;3-1(4)2(5)6;/h5-8H,1-4H2;(H,3,4)(H,5,6);/q-2;;+2/t5-,6-;;/m1../s1 |
InChI Key |
DRMCATBEKSVAPL-BNTLRKBRSA-N |
SMILES |
C1CCC(C(C1)[NH-])[NH-].C(=O)(C(=O)[O-])[O-].[Pt+4] |
Isomeric SMILES |
C1CC[C@H]([C@@H](C1)[NH-])[NH-].C(=O)(C(=O)O)O.[Pt+2] |
Canonical SMILES |
C1CCC(C(C1)[NH-])[NH-].C(=O)(C(=O)O)O.[Pt+2] |
Appearance |
white solid powder |
boiling_point |
100ºC |
| 63121-00-6 61825-94-3 |
|
physical_description |
Solid |
Pictograms |
Irritant; Health Hazard |
Purity |
>98% (or refer to the Certificate of Analysis) |
Related CAS |
63121-00-6 63121-00-6 (SP-4-2 (trans)) |
shelf_life |
>10 years if stored properly |
solubility |
Soluble in water at 4 mg/mL and DMSO at 20 mg/mL; slightly soluble in methanol; insoluble in ethanol. |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
1,2 Diaminocyclohexane Platinum Oxalate 1,2-diaminocyclohexane platinum oxalate 1,2-diamminocyclohexane(trans-1)oxolatoplatinum(II) ACT 078 ACT-078 ACT078 cis-oxalato-(trans-l)-1,2-diaminocyclohexane-platinum(II) Eloxatin Eloxatine L-OHP cpd oxalato-(1,2-cyclohexanediamine)platinum II oxaliplatin oxaliplatin, (SP-4-2-(1R-trans))-isomer oxaliplatin, (SP-4-2-(1S-trans))-isomer oxaliplatin, (SP-4-3-(cis))-isomer oxaliplatine Platinum(2+) ethanedioate (1R,2R)-1,2-cyclohexanediamine (1:1:1) platinum(II)-1,2-cyclohexanediamine oxalate |
Origin of Product |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.