Triclabendazole
Overview
Description
Triclabendazole is a benzimidazole derivative used primarily as an anthelmintic agent to treat infections caused by liver flukes, specifically Fasciola hepatica and Fasciola gigantica . It is marketed under brand names such as Egaten and Fasinex . This compound is unique among benzimidazoles due to its efficacy against both immature and mature stages of liver flukes .
Mechanism of Action
Target of Action
Triclabendazole is an anthelmintic drug primarily used to treat fascioliasis, a parasitic infection often caused by the helminths, Fasciola hepatica and Fasciola gigantica . These parasites, also known as “the common liver fluke” or “the sheep liver fluke”, can infect humans following ingestion of larvae in contaminated water or food .
Mode of Action
This compound and its metabolites are active against both the immature and mature worms of Fasciola hepatica and Fasciola gigantica . The drug and its active metabolites are absorbed by the tegument of the immature and mature worms, leading to a decrease of the resting membrane potential, inhibition of motility, and disruption of the surface as well as ultrastructure that include inhibition of spermatogenesis and vitelline cells .
Biochemical Pathways
This compound is a member of the benzimidazoles and is generally accepted to bind to beta-tubulin, therefore preventing the polymerization of microtubules . This inhibition of tubulin polymerization and protein and enzyme synthesis disrupts the cell’s structural and functional capacity . Additionally, this compound has been found to decrease the intracellular level of cyclic AMP by inhibiting adenylyl cyclase .
Pharmacokinetics
Following a single 10-mg/kg dose of oral this compound given with a meal, peak plasma concentrations of unchanged drug and active sulfoxide metabolite (this compound sulfoxide) are attained within 3–4 hours . Food enhances the absorption of this compound , indicating that its bioavailability is influenced by dietary intake.
Result of Action
The result of this compound’s action is the effective treatment of fascioliasis. The drug’s interaction with its targets leads to the death of the parasites, thereby curing the infection . This compound has been shown in clinical studies to be effective in the treatment of chronic and acute forms of fascioliasis and in both F. hepatica and F. gigantica infections .
Action Environment
The efficacy of this compound can be influenced by environmental factors. For instance, the drug is more effective when administered with food, which enhances its absorption . Furthermore, the prevalence of fascioliasis is higher in areas with contaminated water or food, indicating that environmental sanitation plays a crucial role in the control and prevention of the disease .
Biochemical Analysis
Biochemical Properties
Triclabendazole and its metabolites are active against both the immature and mature worms of Fasciola hepatica and Fasciola gigantica helminths . It is mainly metabolized by the CYP1A2 enzyme into its active sulfoxide metabolite and to a lesser extent by CYP2C9, CYP2C19, CYP2D6, CYP3A, and FMO (flavin containing monooxygenase) .
Cellular Effects
This compound has been found to induce lytic cell death in MCF-7 and MDA-MB-231 breast cancer cells, a typical sign of pyroptosis . It activates apoptosis by regulating the apoptotic protein levels including Bax, Bcl-2, and enhanced cleavage of caspase-8/9/3/7 and PARP . In addition, enhanced cleavage of GSDME was also observed, which indicates the secondary necrosis/pyroptosis is further induced by active caspase-3 .
Molecular Mechanism
The molecular mode of action of this compound consists in binding to beta-tubulin, therefore preventing the polymerization of microtubules . This disrupts the structural integrity of the helminths, leading to their death .
Temporal Effects in Laboratory Settings
In a study on 350 individuals with metabolic syndrome high-risk, after a 3-month proactive intervention, two-thirds of the phenotypic markers were significantly improved in the cohort . This suggests that this compound has a time-dependent effect on biochemical markers.
Dosage Effects in Animal Models
In veterinary medicine, this compound is typically administered at an oral dose of 10 or 12 mg/kg body weight to sheep and cattle, respectively . The effects of this compound vary with different dosages in animal models. For example, in a study on sheep naturally infected with Fasciola sp., treatment with this compound resulted in significant reduction in fecal egg count .
Metabolic Pathways
This compound is metabolized within the host, principally into its sulphoxide and sulphone metabolites . This biotransformation is carried out by the flavin monooxygenase (FMO) and cytochrome P450 (CYP 450) enzyme systems .
Transport and Distribution
This compound and its metabolites are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential . This suggests that this compound is transported and distributed within cells and tissues via absorption.
Subcellular Localization
Given its mechanism of action, it is likely that this compound and its metabolites localize to regions where beta-tubulin is abundant, such as the cytoskeleton of cells .
Preparation Methods
Synthetic Routes and Reaction Conditions
Triclabendazole can be synthesized using various methods. One common method involves starting with 1,2,3-trichlorobenzene, which undergoes hydrolysis in high-concentration alkali liquor to form 2,3-dichlorophenol sodium . This intermediate reacts with 4,5-dichloro-2-nitroaniline in a methylbenzene aqueous solution to form 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline . The nitro group is then reduced using a hydrogen catalytic transfer method, and the resulting compound undergoes methylation to yield this compound .
Another method involves using 3,4-dichloroaniline as the starting material, followed by acylation, nitration, hydrolysis, condensation, reduction with hydrazine hydrate, and ring-closure with S-methylisothiourea sulfate . This method avoids the use of hazardous reagents and high-pressure reactions, making it safer and more environmentally friendly .
Industrial Production Methods
Industrial production of this compound typically follows the synthetic routes mentioned above, with optimizations for large-scale manufacturing. The use of inexpensive and readily available starting materials, along with environmentally friendly reagents, makes the process cost-effective and suitable for large-scale production .
Chemical Reactions Analysis
Types of Reactions
Triclabendazole undergoes various chemical reactions, including:
Oxidation: This compound is metabolized in the liver to form sulfone and sulfoxide metabolites.
Reduction: The nitro group in the intermediate compound is reduced to an amine group during synthesis.
Substitution: The synthesis involves nucleophilic aromatic substitution reactions to introduce the dichlorophenoxy group.
Common Reagents and Conditions
Oxidation: Liver enzymes catalyze the oxidation of this compound to its metabolites.
Reduction: Hydrogen catalytic transfer or hydrazine hydrate is used for the reduction of the nitro group
Substitution: High-concentration alkali liquor and methylbenzene aqueous solution are used for nucleophilic aromatic substitution.
Major Products Formed
Sulfone and sulfoxide metabolites: Formed during the oxidation of this compound in the liver.
4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline: An intermediate in the synthesis of this compound.
Scientific Research Applications
Triclabendazole has a wide range of scientific research applications:
Chemistry: Used as a model compound to study benzimidazole derivatives and their chemical properties.
Biology: Investigated for its effects on liver flukes and other parasitic organisms.
Medicine: Primarily used to treat fascioliasis and paragonimiasis in humans and animals It is the only FDA-approved drug for fascioliasis in humans.
Industry: Used in veterinary medicine to treat liver fluke infections in livestock.
Comparison with Similar Compounds
Triclabendazole is unique among benzimidazoles due to its efficacy against both immature and mature liver flukes . Similar compounds include:
Albendazole: Used to treat a variety of parasitic infections but less effective against liver flukes.
Thiabendazole: Another benzimidazole derivative with a different mechanism of action, primarily used to treat strongyloidiasis.
Closantel: Effective against immature liver flukes but not as broad-spectrum as this compound.
This compound’s unique structure, including a chlorinated benzene ring and the absence of a carbamate group, contributes to its distinct mechanism of action and efficacy .
Properties
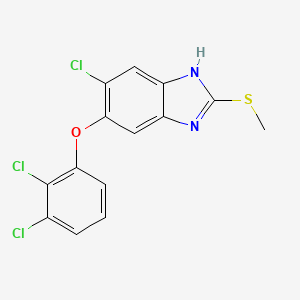
IUPAC Name |
6-chloro-5-(2,3-dichlorophenoxy)-2-methylsulfanyl-1H-benzimidazole | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H9Cl3N2OS/c1-21-14-18-9-5-8(16)12(6-10(9)19-14)20-11-4-2-3-7(15)13(11)17/h2-6H,1H3,(H,18,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NQPDXQQQCQDHHW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CSC1=NC2=CC(=C(C=C2N1)Cl)OC3=C(C(=CC=C3)Cl)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H9Cl3N2OS | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7043952 | |
| Record name | Triclabendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7043952 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
359.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
0.5 [ug/mL] (The mean of the results at pH 7.4) | |
| Record name | SID50085431 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Triclabendazole is an anthelmintic agent against _Fasciola_ species. The mechanism of action against Fasciola species is not fully understood at this time. In vitro studies and animal studies suggest that triclabendazole and its active metabolites (_sulfoxide_ and _sulfone_) are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential, the inhibition of tubulin function as well as protein and enzyme synthesis necessary for survival. These metabolic disturbances lead to an inhibition of motility, disruption of the worm outer surface, in addition to the inhibition of spermatogenesis and egg/embryonic cells. **A note on resistance** In vitro studies, in vivo studies, as well as case reports suggest a possibility for the development of resistance to triclabendazole. The mechanism of resistance may be multifactorial and include changes in drug uptake/efflux mechanisms, target molecules, and changes in drug metabolism. The clinical significance of triclabendazole resistance in humans is not yet elucidated. | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
68786-66-3 | |
| Record name | Triclabendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=68786-66-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Triclabendazole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0068786663 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Triclabendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759250 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Triclabendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7043952 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1H-Benzimidazole, 6-chloro-5-(2,3-dichlorophenoxy)-2-(methylthio) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.414 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRICLABENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4784C8E03O | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
189-191 | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.