Ertugliflozin
Overview
Description
Ertugliflozin is a selective inhibitor of sodium-glucose cotransporter 2 (SGLT2), primarily used for the treatment of type 2 diabetes mellitus. It works by blocking the reabsorption of glucose in the kidneys, leading to increased glucose excretion through urine. This helps in lowering blood glucose levels in patients with type 2 diabetes .
Mechanism of Action
Target of Action
Ertugliflozin is a selective inhibitor of the sodium-glucose cotransporter 2 (SGLT2) . SGLT2 is primarily found in the proximal convoluted tubules in the kidneys . It plays a crucial role in glucose homeostasis by facilitating the reabsorption of approximately 90% of glucose from renal tubules .
Mode of Action
This compound works by blocking the SGLT2 . This inhibition prevents glucose reabsorption from the glomerulus, leading to an increase in urinary glucose excretion . As a result, blood glucose levels decrease, which is beneficial for managing type 2 diabetes mellitus .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the glucose reabsorption pathway in the kidneys . By inhibiting SGLT2, this compound disrupts this pathway, leading to increased glucose excretion in the urine and decreased blood glucose levels . Additionally, this compound has been shown to cause a switch in cardiac substrate utilization, leading to reduced cardiac mTOR-signaling, unfolded protein response, and apoptosis .
Result of Action
The primary molecular effect of this compound is the reduction of blood glucose levels . At the cellular level, this compound reduces the reabsorption of glucose in the kidneys, leading to increased glucose excretion . This results in improved glycemic control in patients with type 2 diabetes mellitus . Furthermore, this compound has been shown to reduce cardiac mTOR-signaling, unfolded protein response, and apoptosis .
Action Environment
Environmental factors such as diet, exercise, and the presence of other medications can influence the action of this compound . For instance, administration of this compound with food resulted in no meaningful effect on this compound area under the plasma concentration–time curve (AUC), but decreased peak concentrations (Cmax) by 29% .
Biochemical Analysis
Biochemical Properties
Ertugliflozin plays a crucial role in biochemical reactions by inhibiting the sodium-glucose cotransporter 2, which is predominantly expressed in the proximal renal tubules. This inhibition prevents the reabsorption of glucose from the glomerular filtrate back into the bloodstream, thereby increasing urinary glucose excretion . This compound interacts with several biomolecules, including the sodium-glucose cotransporter 2 protein itself. The binding of this compound to sodium-glucose cotransporter 2 is highly selective, ensuring minimal interaction with other glucose transporters .
Cellular Effects
This compound exerts significant effects on various cell types, particularly renal tubular cells. By inhibiting sodium-glucose cotransporter 2, this compound reduces glucose reabsorption, leading to increased glucose excretion and decreased blood glucose levels . This reduction in glucose levels can influence cell signaling pathways, such as the insulin signaling pathway, and can lead to changes in gene expression related to glucose metabolism . Additionally, this compound has been shown to reduce oxidative stress and inflammation in renal cells, contributing to its protective effects on kidney function .
Molecular Mechanism
The molecular mechanism of action of this compound involves the selective inhibition of sodium-glucose cotransporter 2. This compound binds to the sodium-glucose cotransporter 2 protein, blocking its ability to transport glucose and sodium ions across the renal tubular cell membrane . This inhibition leads to increased glucose excretion in the urine and decreased blood glucose levels. This compound also affects the expression of genes involved in glucose metabolism and insulin signaling, further contributing to its hypoglycemic effects .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to change over time. This compound is rapidly absorbed following oral administration, with peak plasma concentrations occurring within 1 to 2 hours . The terminal elimination half-life of this compound ranges from 11 to 18 hours, allowing for once-daily dosing . Long-term studies have shown that this compound maintains its efficacy in reducing blood glucose levels over extended periods, with minimal degradation or loss of stability . Additionally, this compound has been shown to have long-term protective effects on renal function in both in vitro and in vivo studies .
Dosage Effects in Animal Models
In animal models, the effects of this compound vary with different dosages. Studies have shown that lower doses of this compound effectively reduce blood glucose levels without causing significant adverse effects . Higher doses of this compound can lead to increased urinary glucose excretion and potential adverse effects, such as dehydration and electrolyte imbalances . Threshold effects have been observed, with a plateau in glucose-lowering effects at higher doses . Toxicity studies in animal models have shown that this compound is generally well-tolerated, with a low incidence of adverse effects at therapeutic doses .
Metabolic Pathways
This compound is primarily metabolized through glucuronidation, mediated by the enzymes uridine 5’-diphospho-glucuronosyltransferase 1A9 and uridine 5’-diphospho-glucuronosyltransferase 2B7 . Approximately 86% of this compound is metabolized via this pathway, resulting in the formation of pharmacologically inactive glucuronide conjugates . A minor portion of this compound undergoes oxidative metabolism by cytochrome P450 3A4, contributing to about 12% of its clearance . The metabolic pathways of this compound ensure its efficient elimination from the body, with minimal accumulation of active drug .
Transport and Distribution
This compound is rapidly absorbed following oral administration, with peak plasma concentrations occurring within 1 to 2 hours . The drug is widely distributed throughout the body, with a volume of distribution of approximately 85 liters . This compound is highly bound to plasma proteins, primarily albumin, which facilitates its transport and distribution within the bloodstream . The primary route of elimination for this compound is through metabolism, with minimal renal excretion of the unchanged drug .
Subcellular Localization
This compound primarily localizes to the renal proximal tubular cells, where it exerts its inhibitory effects on sodium-glucose cotransporter 2 . The drug’s localization to these cells is facilitated by its high affinity for the sodium-glucose cotransporter 2 protein, ensuring targeted inhibition of glucose reabsorption . This compound does not undergo significant post-translational modifications or targeting signals that direct it to other cellular compartments or organelles .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of ertugliflozin involves multiple steps, starting with the protection of the primary alcohol moiety of an intermediate compound with a trityl group in the presence of pyridine. This is followed by subsequent deprotection with p-toluenesulfonic acid . The process also involves the use of compounds of Formula III, Formula IV, and Formula VII, which are protected with suitable protecting groups to ensure high purity and yield .
Industrial Production Methods: Industrial production of this compound follows similar synthetic routes but on a larger scale. The process involves the use of advanced techniques and equipment to ensure high yield and purity. The use of hazardous chemicals like pyridine is minimized to ensure safety and compliance with industrial standards .
Chemical Reactions Analysis
Types of Reactions: Ertugliflozin undergoes various chemical reactions, including oxidation, reduction, and substitution. It is stable under acidic, alkaline, and thermal conditions but shows notable degradation under photolytic and oxidative conditions .
Common Reagents and Conditions: Common reagents used in the reactions involving this compound include p-toluenesulfonic acid for deprotection and orthophosphoric acid for pH adjustment. The reactions are typically carried out under controlled conditions to ensure the stability and purity of the compound .
Major Products Formed: The major products formed from the reactions involving this compound include its metabolites, which are primarily excreted through urine. The primary metabolic pathway is glucuronidation, accounting for approximately 86% of the metabolism .
Scientific Research Applications
Ertugliflozin has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry. In medicine, it is used to manage type 2 diabetes mellitus by improving glycemic control. It has also been studied for its potential cardiovascular benefits, including reducing the risk of major adverse cardiovascular events and hospitalizations for heart failure . In chemistry, it is used as a model compound for studying the pharmacokinetics and pharmacodynamics of SGLT2 inhibitors . In industry, it is used in the development of new antidiabetic medications and formulations .
Comparison with Similar Compounds
Ertugliflozin is part of the SGLT2 inhibitor class of medications, which also includes canagliflozin, dapagliflozin, and empagliflozin. Compared to these compounds, this compound has shown similar efficacy in lowering blood glucose levels but may have a different safety and side effect profile . For instance, this compound has been found to have a lower risk of causing urinary tract infections compared to some other SGLT2 inhibitors .
List of Similar Compounds:- Canagliflozin
- Dapagliflozin
- Empagliflozin
- Bexagliflozin
Properties
IUPAC Name |
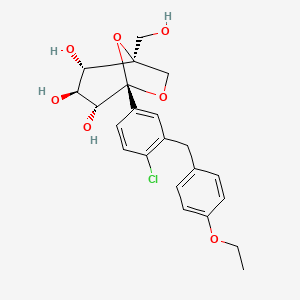
(1S,2S,3S,4R,5S)-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
MCIACXAZCBVDEE-CUUWFGFTSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)C34C(C(C(C(O3)(CO4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)[C@@]34[C@@H]([C@H]([C@@H]([C@@](O3)(CO4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H25ClO7 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40153120 | |
| Record name | PF-04971729 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40153120 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
436.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Very slightly soluble | |
| Record name | Ertugliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11827 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
As part of a normal process, the glucose from the blood is filtered for excretion and reabsorbed in the glomerulus so less than one percent of this glucose is excreted in the urine. The reabsorption is mediated by the sodium-dependent glucose cotransporter (SGLT), mainly the type 2 which is responsible for 90% of the reabsorbed glucose. Ertugliflozin is a small inhibitor of the SGLT2 and its activity increases glucose excretion, reducing hyperglycemia without the requirement of excessive insulin secretion. | |
| Record name | Ertugliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11827 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1210344-57-2, 1431329-06-4, 1210344-83-4 | |
| Record name | Ertugliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1210344-57-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ertugliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1210344572 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ertugliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11827 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PF-04971729 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40153120 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1,6-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-5-C-(hydroxymethyl)-beta-L-idopyranose | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Ertugliflozin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Ertugliflozin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Ertugliflozin | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/6C282481IP | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


![(3As,4r,10as)-2,6-diamino-10,10-dihydroxy-4-(hydroxymethyl)-3a,4,9,10-tetrahydro-3h,8h-pyrrolo[1,2-c]purin-9-yl hydrogen sulfate](/img/structure/B560001.png)
![Sodium ((1,4-phenylenebis(methanylylidene))bis(7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl-3-ylidene))dimethanesulfonate](/img/structure/B560017.png)
![[[(2R,3R,4R,5R)-5-(6-aminopurin-9-yl)-3-hydroxy-4-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate;cyclohexanamine](/img/structure/B560022.png)