那格列奈
描述
那格列奈是一种口服降糖药,用于治疗2型糖尿病。它属于降血糖药物中的甲磺酰脲类。 那格列奈由日本公司味之素开发,由诺华以商品名Starlix上市 .
作用机制
那格列奈通过刺激胰腺释放胰岛素来降低血糖水平。它通过关闭β细胞膜上的ATP依赖性钾通道来实现这一点。这会使β细胞去极化,导致电压依赖性钙通道打开。 由此产生的钙离子内流会诱导含有胰岛素的囊泡与细胞膜融合,导致胰岛素分泌 .
科学研究应用
那格列奈有几个科学研究应用:
药物研究: 那格列奈用于开发缓释制剂,以提高其生物利用度和治疗效果.
生物学研究: 那格列奈用于研究胰岛素分泌和葡萄糖代谢机制的研究.
工业应用: 那格列奈用于快速崩解片的制剂,以提高患者依从性.
生化分析
Biochemical Properties
Nateglinide plays a crucial role in biochemical reactions by interacting with specific enzymes and proteins. It primarily targets the ATP-sensitive potassium channels (K_ATP channels) on the pancreatic beta cells. By binding to these channels, Nateglinide inhibits their activity, leading to cell membrane depolarization. This depolarization opens voltage-gated calcium channels, resulting in an influx of calcium ions. The increased intracellular calcium concentration triggers the exocytosis of insulin-containing vesicles, thereby increasing insulin secretion .
Cellular Effects
Nateglinide influences various cellular processes, particularly in pancreatic beta cells. It enhances insulin secretion, which is crucial for glucose homeostasis. Additionally, Nateglinide affects cell signaling pathways by modulating the activity of K_ATP channels and calcium channels. This modulation impacts gene expression related to insulin synthesis and secretion. In other cell types, Nateglinide’s effects are less pronounced but may include alterations in cellular metabolism and signaling pathways .
Molecular Mechanism
At the molecular level, Nateglinide exerts its effects by binding to the sulfonylurea receptor 1 (SUR1) subunit of the K_ATP channels on pancreatic beta cells. This binding inhibits the channel’s activity, leading to membrane depolarization and subsequent opening of voltage-gated calcium channels. The resulting calcium influx promotes insulin vesicle fusion with the cell membrane and insulin release. Nateglinide’s rapid binding and dissociation from the SUR1 subunit contribute to its short duration of action .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Nateglinide have been observed to change over time. The compound is relatively stable under standard conditions but can degrade under extreme pH or temperature conditions. Long-term studies have shown that Nateglinide maintains its efficacy in stimulating insulin secretion over extended periods, although its rapid onset and short duration of action mean that its effects are most pronounced shortly after administration .
Dosage Effects in Animal Models
In animal models, the effects of Nateglinide vary with dosage. At therapeutic doses, Nateglinide effectively lowers postprandial blood glucose levels without causing significant hypoglycemia. At higher doses, adverse effects such as hypoglycemia and potential toxicity have been observed. These effects highlight the importance of careful dosage management to maximize therapeutic benefits while minimizing risks .
Metabolic Pathways
Nateglinide is metabolized primarily in the liver through the cytochrome P450 enzyme system, particularly CYP2C9 and CYP3A4. The major metabolites are less active than the parent compound and are excreted in urine and feces. Nateglinide’s metabolism involves hydroxylation and subsequent conjugation reactions, which facilitate its elimination from the body .
Transport and Distribution
Within cells, Nateglinide is transported and distributed through passive diffusion and active transport mechanisms. It binds to plasma proteins, which influences its distribution and bioavailability. Nateglinide’s rapid absorption and distribution are critical for its quick onset of action, allowing it to effectively manage postprandial glucose levels .
Subcellular Localization
Nateglinide’s subcellular localization is primarily within the cytoplasm of pancreatic beta cells, where it interacts with K_ATP channels on the cell membrane. This localization is essential for its role in modulating insulin secretion. Nateglinide does not require specific targeting signals or post-translational modifications for its activity, as its effects are mediated through direct binding to its target channels .
准备方法
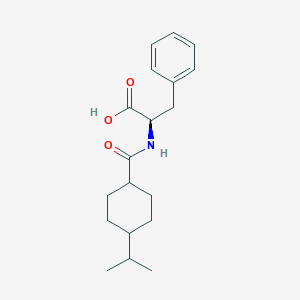
那格列奈可以通过多种方法合成。一种常见的方法是将D-苯丙氨酸与反式-4-异丙基环己烷羧酸反应。 反应通常在有机溶剂中使用偶联剂(如二环己基碳二亚胺 (DCC))和催化剂(如4-二甲氨基吡啶 (DMAP))存在下进行 . 另一种方法是使用油包水 (O/W) 溶剂乳化技术制备负载那格列奈的缓释乙基纤维素微球 .
化学反应分析
相似化合物的比较
属性
IUPAC Name |
(2R)-3-phenyl-2-[(4-propan-2-ylcyclohexanecarbonyl)amino]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H27NO3/c1-13(2)15-8-10-16(11-9-15)18(21)20-17(19(22)23)12-14-6-4-3-5-7-14/h3-7,13,15-17H,8-12H2,1-2H3,(H,20,21)(H,22,23)/t15?,16?,17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OELFLUMRDSZNSF-OFLPRAFFSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C1CCC(CC1)C(=O)NC(CC2=CC=CC=C2)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)C1CCC(CC1)C(=O)N[C@H](CC2=CC=CC=C2)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H27NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9040687 | |
| Record name | Nateglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9040687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
317.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble | |
| Record name | Nateglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00731 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Nateglinide activity is dependent on the presence functioning β cells and glucose. In contrast to sulfonylurea insulin secretatogogues, nateglinide has no effect on insulin release in the absence of glucose. Rather, it potentiates the effect of extracellular glucose on ATP-sensitive potassium channel and has little effect on insulin levels between meals and overnight. As such, nateglinide is more effective at reducing postprandial blood glucose levels than fasting blood glucose levels and requires a longer duration of therapy (approximately one month) before decreases in fasting blood glucose are observed. The insulinotropic effects of nateglinide are highest at intermediate glucose levels (3 to 10 mmol/L) and it does not increase insulin release already stimulated by high glucose concentrations (greater than 15 mmol/L). Nateglinide appears to be selective for pancreatic β cells and does not appear to affect skeletal or cardiac muscle or thyroid tissue. | |
| Record name | Nateglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00731 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
105816-04-4, 105816-06-6 | |
| Record name | Nateglinide [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0105816044 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | N-((cis-4-(1-Methylethyl)cyclohexyl)carbonyl)-D-phenylalanine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0105816066 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nateglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00731 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nateglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9040687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | NATEGLINIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/41X3PWK4O2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | N-((CIS-4-(1-METHYLETHYL)CYCLOHEXYL)CARBONYL)-D-PHENYLALANINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/XTM4DQP5S5 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![(6R,7S)-7-(Benzoylamino)-3-methylene-8-oxo-5-oxa-1-azabicyclo[4.2.0]octane-2-carboxylic Acid Diphenylmethyl Ester](/img/structure/B44563.png)