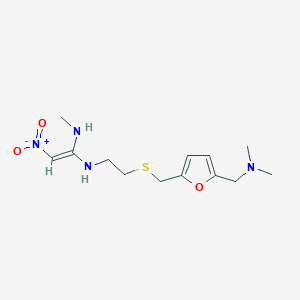
Ranitidin
Übersicht
Beschreibung
Ranitidine is a histamine H2-receptor antagonist that was widely used to decrease stomach acid production. It was commonly prescribed for the treatment of peptic ulcer disease, gastroesophageal reflux disease, and Zollinger-Ellison syndrome. Ranitidine was discovered in England in 1976 and came into commercial use in 1981. It was marketed under the brand name Zantac, among others .
Wirkmechanismus
Target of Action
Ranitidine is a histamine H2-receptor antagonist . These receptors are found on the gastric parietal cells in the stomach . The primary role of these receptors is to mediate the release of gastric acid .
Mode of Action
Ranitidine works by blocking histamine . This results in a decrease in the amount of acid released by the cells of the stomach . It achieves this by reversibly binding to the histamine H2 receptors, which inhibits histamine binding to this receptor, thereby reducing gastric acid secretion .
Biochemical Pathways
The biochemical pathway affected by ranitidine involves the hormone gastrin. After a meal, gastrin is produced by cells in the lining of the stomach. Gastrin stimulates the release of histamine, which then binds to histamine H2 receptors, leading to the secretion of gastric acid . By blocking these receptors, ranitidine reduces the secretion of gastric acid .
Pharmacokinetics
Ranitidine has a bioavailability of 50% when administered orally . It is metabolized in the liver by FMOs, including FMO3, among other enzymes . The elimination half-life of ranitidine is between 2-3 hours, and it is excreted 30-70% through the kidneys .
Result of Action
The molecular and cellular effects of ranitidine’s action result in a decrease in gastric acid secretion . This helps to prevent and treat gastric-acid associated conditions, including ulcers . It is also used to treat conditions where the stomach produces too much acid, such as Zollinger-Ellison syndrome .
Wissenschaftliche Forschungsanwendungen
Ranitidin wurde ausgiebig auf seine Anwendungen in verschiedenen Bereichen untersucht:
Chemie: Die chemischen Eigenschaften und Reaktionen von this compound wurden zur Entwicklung neuer Synthesemethoden und zum Verständnis seiner Abbauwege untersucht.
Biologie: this compound wurde in Studien zu seinen Auswirkungen auf Histaminrezeptoren und seiner Rolle bei der Reduzierung der Magensäuresekretion eingesetzt.
Medizin: this compound wurde weit verbreitet zur Behandlung von Erkrankungen wie Magengeschwüren, gastroösophagealer Refluxkrankheit und Zollinger-Ellison-Syndrom eingesetzt.
5. Wirkmechanismus
This compound wirkt, indem es Histamin-H2-Rezeptoren in der Magenschleimhaut blockiert. Histamin, das von enterochromaffinartigen Zellen freigesetzt wird, bindet an diese Rezeptoren und stimuliert die Sekretion von Magensäure. Indem es diese Rezeptoren blockiert, reduziert this compound die Produktion von Magensäure und lindert so Symptome, die mit überschüssiger Magensäure verbunden sind .
Biochemische Analyse
Biochemical Properties
Ranitidine works by blocking the action of histamine on the H2 receptors of the parietal cells in the stomach, thereby reducing the production of stomach acid. The compound interacts with these receptors, preventing histamine from binding and triggering acid production .
Cellular Effects
Ranitidine’s primary effect on cells is the reduction of gastric acid secretion in parietal cells. This can influence various cellular processes, including the regulation of intracellular pH and the activation of certain enzymes that require an acidic environment .
Molecular Mechanism
The molecular mechanism of Ranitidine involves its binding to H2 receptors on the parietal cells of the stomach. This prevents histamine from binding to these receptors and triggering the secretion of gastric acid. This action does not involve enzyme inhibition or activation, but rather receptor antagonism .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Ranitidine are observed to be relatively stable over time. The drug does not undergo significant degradation and continues to exert its acid-suppressing effects as long as it is present in the system .
Dosage Effects in Animal Models
In animal models, the effects of Ranitidine have been observed to be dose-dependent. Higher doses result in greater suppression of gastric acid secretion. Extremely high doses may lead to adverse effects, although these are generally rare .
Metabolic Pathways
Ranitidine is metabolized in the liver through the cytochrome P450 system. It does not significantly interact with or alter other metabolic pathways .
Transport and Distribution
After oral administration, Ranitidine is absorbed in the gastrointestinal tract and distributed throughout the body. It can cross cell membranes and reach its site of action in the stomach .
Subcellular Localization
Ranitidine acts on the cell surface, specifically on the H2 receptors of parietal cells in the stomach. It does not have a specific subcellular localization as its site of action is on the cell surface .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Ranitidin kann über mehrere Wege synthetisiert werden. Ein übliches Verfahren beinhaltet das Zwischenprodukt 5-(Dimethylamino)furfurylthioethylamin. Die Synthese beginnt mit Furfurylalkohol, der einer Reihe von Reaktionen unterzogen wird, um das Zwischenprodukt zu bilden. Dieses Zwischenprodukt wird dann mit l-Methylthio-l-(N-Methylamino)-2-Nitroethylen umgesetzt, um this compound zu produzieren .
Industrielle Produktionsmethoden: Die industrielle Produktion von this compound erfolgt in der Regel unter Verwendung organischer Lösungsmittel und moderater Reaktionsbedingungen. Beispielsweise kann die Verbindung synthetisiert werden, indem ein Zwischenprodukt mit N,N-Dimethylaminotriphenylphosphoniumsalzen und Dimethylamin bei etwa 90 °C in organischen Lösungsmitteln wie Dimethylformamid behandelt wird .
Analyse Chemischer Reaktionen
Reaktionstypen: Ranitidin unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann unter bestimmten Bedingungen oxidiert werden, was zur Bildung verschiedener Nebenprodukte führt.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Oxidationsmittel können zur Oxidation von this compound verwendet werden.
Photolyse: Photolysereaktionen erfordern in der Regel Lichteinwirkung und können durch das Vorhandensein von natürlicher organischer Substanz beeinflusst werden.
Hauptprodukte, die gebildet werden:
Oxidation: Es können verschiedene Oxidationsprodukte gebildet werden, abhängig von den spezifischen Bedingungen und Reagenzien, die verwendet werden.
Photolyse: Photolyse von this compound kann zur Bildung mehrerer Abbauprodukte führen.
Vergleich Mit ähnlichen Verbindungen
Ranitidin gehört zur Klasse der Histamin-H2-Rezeptor-Antagonisten, zu denen auch Verbindungen wie Cimetidin und Famotidin gehören.
Ähnliche Verbindungen:
Cimetidin: Der erste entdeckte H2-Rezeptor-Antagonist. Er hat einen ähnlichen Wirkmechanismus, aber eine andere chemische Struktur.
Einzigartigkeit von this compound: this compound wurde aufgrund seines verbesserten Nebenwirkungsprofils und seiner Potenz gegenüber Cimetidin bevorzugt. Bedenken hinsichtlich des Vorhandenseins von N-Nitrosodimethylamin in Ranitidinprodukten haben zu seinem Rückzug aus vielen Märkten geführt .
Eigenschaften
IUPAC Name |
(E)-1-N'-[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3/b13-9+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VMXUWOKSQNHOCA-UKTHLTGXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CNC(=C[N+](=O)[O-])NCCSCC1=CC=C(O1)CN(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN/C(=C\[N+](=O)[O-])/NCCSCC1=CC=C(O1)CN(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C13H22N4O3S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID101112063 | |
| Record name | (1E)-N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101112063 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
314.41 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ranitidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001930 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Water soluble | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
H2 antagonists inhibit gastric acid secretion elicited by histamine and other H2 agonists in a dose dependent, competitive manner; the degree of inhibition parallels the concentration of the drug in plasma over a wide range. The H2 antagonists also inhibit acid secretion elicited by gastrin and, to a lesser extent, by muscarinic agonists. Importantly, these drugs inhibit basal (fasting) and nocturnal acid secretion and that stimulated by food, sham feeding, fundic distention, and various pharmacological agents; this property reflects the vital role of histamine in mediating the effects of diverse stimuli. /H2 Receptor Antagonists/, ... /H2 Antagonists/ measurably inhibit effects on the cardiovascular and other systems that are elicited through H2 receptors by exogenous or endogenous histamine. /H2 Receptor Antagonists/, ...IS A COMPETITIVE ANTAGONIST OF HISTAMINE-INDUCED GASTRIC ACID SECRETION... INHIBITS BOTH THE VOLUME AND CONCENTRATION OF GASTRIC ACID INDUCED NOCTURNALLY AND BY FOOD BUT DOES NOT AFFECT GASTRIC MUCUS OR ITS PRODUCTION. ...DOES NOT AFFECT LOWER ESOPHAGEAL SPHINCTER PRESSURE... | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
SOLID | |
CAS No. |
82530-72-1, 66357-35-5 | |
| Record name | (1E)-N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=82530-72-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | ranitidine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757851 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | (1E)-N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101112063 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Ranitidine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.060.283 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ranitidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001930 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
69-70 °C, MP: 133-134 °C /RATINIDINE HYDROCHLORIDE/ | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does ranitidine exert its therapeutic effect?
A: Ranitidine acts as a competitive antagonist at histamine H2 receptors found on the basolateral membrane of parietal cells in the stomach. [] By blocking histamine binding to these receptors, ranitidine effectively reduces the secretion of gastric acid, providing relief from symptoms associated with hyperacidity. []
Q2: What are the key pharmacokinetic properties of ranitidine?
A: Ranitidine is well absorbed after oral administration, reaching peak plasma concentrations within 1-3 hours. [] It is metabolized in the liver to several metabolites, with the primary metabolite being desmethylranitidine. [] Approximately 77% of an administered dose is excreted unchanged in the urine, with the remainder excreted as metabolites. [] The elimination half-life of ranitidine is 2.9-3.9 hours. []
Q3: Does ranitidine interact with other drugs?
A: Yes, ranitidine has been shown to interact with several drugs, primarily through its effects on drug-metabolizing enzymes in the liver. [] It can inhibit the cytochrome P450 enzyme system, particularly the CYP1A2 and CYP2D6 isoenzymes. [] This inhibition can lead to increased plasma concentrations of drugs that are metabolized by these enzymes, potentially resulting in adverse effects.
Q4: What are the safety concerns associated with ranitidine use?
A: While generally well-tolerated, ranitidine has been associated with rare but potentially serious adverse effects, including hypersensitivity reactions, hematological abnormalities, and hepatic dysfunction. [, ] Furthermore, the detection of N-nitrosodimethylamine (NDMA), a probable human carcinogen, in certain ranitidine formulations has raised concerns about potential long-term risks. []
Q5: What formulations of ranitidine are available?
A: Ranitidine is available in various formulations, including oral tablets, effervescent tablets, syrups, and solutions for intravenous administration. [] The choice of formulation depends on the patient's age, medical condition, and preference.
Q6: What are the main therapeutic applications of ranitidine?
A6: Ranitidine was widely prescribed for conditions associated with gastric hyperacidity, such as:
- Duodenal and gastric ulcers: Clinical trials demonstrated the efficacy of ranitidine in promoting ulcer healing and relieving symptoms. [, ]
- Gastroesophageal reflux disease (GERD): Ranitidine effectively reduces heartburn and other symptoms of GERD. []
- Zollinger-Ellison syndrome: This rare condition involves excessive gastric acid production, and ranitidine can help manage symptoms. []
Q7: What alternatives to ranitidine are available for treating acid-related disorders?
A7: Several alternatives to ranitidine are available, including:
- Proton pump inhibitors (PPIs): These drugs, such as omeprazole, lansoprazole, and esomeprazole, are more potent inhibitors of gastric acid secretion than H2-receptor antagonists. []
- Antacids: These over-the-counter medications provide rapid but short-term relief from heartburn and indigestion by neutralizing stomach acid. []
- Alginates: These medications form a protective barrier over the stomach contents, preventing acid reflux into the esophagus. []
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![3-methylimidazo[4,5-f]quinoline](/img/structure/B14863.png)