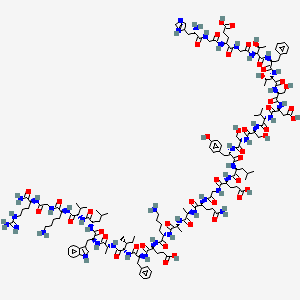
H-His-Gly-Glu-Gly-aThr-Phe-aThr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-D-Ala-D-Ala-Lys-Glu-Phe-aIle-D-Ala-Trp-Leu-Val-Lys-Gly-Arg-NH2
Descripción general
Descripción
Albiglutide es un agonista del receptor del péptido similar al glucagón-1 que se utiliza en el tratamiento de la diabetes mellitus tipo 2. Se comercializa bajo los nombres comerciales Eperzan en Europa y Tanzeum en los Estados Unidos. Albiglutide es un análogo polipeptídico del péptido similar al glucagón-1 humano producido por ADN recombinante, diseñado para mejorar la secreción de insulina dependiente de la glucosa, suprimir la secreción inadecuada de glucagón, retrasar el vaciado gástrico y reducir la ingesta de alimentos .
Mecanismo De Acción
Albiglutide actúa como un agonista en el receptor del péptido similar al glucagón-1. Esta activación del receptor conduce a un aumento en la secreción de insulina dependiente de la glucosa de las células beta pancreáticas. Además, albiglutide suprime la secreción de glucagón, retrasa el vaciado gástrico y promueve la saciedad. Estos efectos combinados ayudan en la regulación de los niveles de glucosa en sangre .
Análisis Bioquímico
Biochemical Properties
Albiglutide acts as an agonist at the GLP-1 receptor, which makes it a type of incretin mimetic . This causes an increase in insulin secretion, predominantly in the presence of high blood glucose, and also slows down gastric emptying .
Cellular Effects
Albiglutide has a significant impact on various types of cells and cellular processes. It influences cell function by increasing insulin secretion, predominantly in the presence of high blood glucose . This can have a profound effect on cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
The molecular mechanism of action of Albiglutide involves its role as an agonist of the GLP-1 receptor . This interaction leads to an increase in insulin secretion, predominantly in the presence of high blood glucose. It also slows down gastric emptying, which can have a significant impact on the body’s metabolic processes .
Temporal Effects in Laboratory Settings
It is known that Albiglutide has a half-life of 5 (4–7) days , indicating its stability and potential for long-term effects on cellular function.
Metabolic Pathways
Albiglutide is involved in the incretin metabolic pathway, where it acts as an agonist of the GLP-1 receptor . This interaction leads to an increase in insulin secretion, predominantly in the presence of high blood glucose, and also slows down gastric emptying .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: Albiglutide se sintetiza utilizando tecnología de ADN recombinante. El proceso implica la inserción del gen que codifica el polipéptido albiglutide en un vector de expresión adecuado, que luego se introduce en una célula huésped, típicamente Escherichia coli o levadura. Las células huésped se cultivan en condiciones específicas para expresar el polipéptido albiglutide, que posteriormente se purifica mediante una serie de técnicas cromatográficas .
Métodos de producción industrial: La producción industrial de albiglutide sigue un enfoque similar de ADN recombinante, pero a mayor escala. El proceso de producción incluye la fermentación, la lisis celular, la extracción de proteínas y la purificación. El producto final se formula en una inyección subcutánea para uso clínico .
Análisis De Reacciones Químicas
Tipos de reacciones: Albiglutide experimenta principalmente degradación proteolítica en el cuerpo. No participa en reacciones químicas típicas como la oxidación, la reducción o la sustitución debido a su naturaleza peptídica .
Reactivos y condiciones comunes: La degradación de albiglutide implica la escisión enzimática por proteasas. Las condiciones específicas para estas reacciones son fisiológicas, ocurriendo dentro del cuerpo humano .
Productos principales formados: Los productos principales formados a partir de la degradación de albiglutide son fragmentos peptídicos más pequeños y aminoácidos, que se metabolizan o excretan posteriormente .
Aplicaciones Científicas De Investigación
Albiglutide tiene varias aplicaciones de investigación científica, particularmente en los campos de la medicina y la farmacología:
Medicina: Albiglutide se utiliza para controlar los niveles de glucosa en sangre en pacientes con diabetes mellitus tipo 2.
Investigación cardiovascular: Albiglutide se ha investigado por sus posibles beneficios cardiovasculares, incluidos sus efectos en la reducción del riesgo de eventos cardiovasculares en pacientes con diabetes tipo 2.
Comparación Con Compuestos Similares
Albiglutide es parte de una clase de fármacos conocidos como agonistas del receptor del péptido similar al glucagón-1. Compuestos similares en esta clase incluyen liraglutide, exenatide, dulaglutide y semaglutide.
Comparación:
Singularidad: La característica única de albiglutide es su fusión con albúmina humana, lo que extiende su vida media y permite una dosificación una vez por semana. Esta fusión también reduce el riesgo de inmunogenicidad en comparación con otros agonistas del receptor del péptido similar al glucagón-1 .
Propiedades
IUPAC Name |
(4S)-5-[[2-[[(2S,3S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-5-amino-1-[[(2R)-1-[[(2R)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3R)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[2-[[(2S)-1-amino-5-carbamimidamido-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-2-oxoethyl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]acetyl]amino]-5-oxopentanoic acid | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C148H224N40O45/c1-16-76(10)119(145(231)166-79(13)125(211)174-103(59-85-62-158-90-35-24-23-34-88(85)90)135(221)176-99(55-73(4)5)136(222)185-117(74(6)7)143(229)173-92(36-25-27-51-149)127(213)160-65-109(196)167-91(122(153)208)38-29-53-157-148(154)155)187-137(223)101(56-82-30-19-17-20-31-82)177-132(218)97(46-50-115(204)205)172-131(217)93(37-26-28-52-150)170-124(210)78(12)164-123(209)77(11)165-130(216)96(43-47-108(152)195)169-111(198)66-161-129(215)95(45-49-114(202)203)171-133(219)98(54-72(2)3)175-134(220)100(58-84-39-41-87(194)42-40-84)178-140(226)105(68-189)181-142(228)107(70-191)182-144(230)118(75(8)9)186-139(225)104(61-116(206)207)179-141(227)106(69-190)183-147(233)121(81(15)193)188-138(224)102(57-83-32-21-18-22-33-83)180-146(232)120(80(14)192)184-112(199)67-162-128(214)94(44-48-113(200)201)168-110(197)64-159-126(212)89(151)60-86-63-156-71-163-86/h17-24,30-35,39-42,62-63,71-81,89,91-107,117-121,158,189-194H,16,25-29,36-38,43-61,64-70,149-151H2,1-15H3,(H2,152,195)(H2,153,208)(H,156,163)(H,159,212)(H,160,213)(H,161,215)(H,162,214)(H,164,209)(H,165,216)(H,166,231)(H,167,196)(H,168,197)(H,169,198)(H,170,210)(H,171,219)(H,172,217)(H,173,229)(H,174,211)(H,175,220)(H,176,221)(H,177,218)(H,178,226)(H,179,227)(H,180,232)(H,181,228)(H,182,230)(H,183,233)(H,184,199)(H,185,222)(H,186,225)(H,187,223)(H,188,224)(H,200,201)(H,202,203)(H,204,205)(H,206,207)(H4,154,155,157)/t76-,77-,78-,79-,80+,81+,89+,91+,92+,93+,94+,95+,96+,97+,98+,99+,100+,101+,102+,103+,104+,105+,106+,107+,117+,118+,119+,120+,121+/m1/s1 | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JYDZPPZAYQTOIV-OTSUTHPESA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC(C)C(C(=O)NC(C)C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCCNC(=N)N)C(=O)N)NC(=O)C(CC3=CC=CC=C3)NC(=O)C(CCC(=O)O)NC(=O)C(CCCCN)NC(=O)C(C)NC(=O)C(C)NC(=O)C(CCC(=O)N)NC(=O)CNC(=O)C(CCC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(CO)NC(=O)C(CO)NC(=O)C(C(C)C)NC(=O)C(CC(=O)O)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)C(C(C)O)NC(=O)CNC(=O)C(CCC(=O)O)NC(=O)CNC(=O)C(CC6=CN=CN6)N | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC[C@@H](C)[C@@H](C(=O)N[C@H](C)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(=N)N)C(=O)N)NC(=O)[C@H](CC3=CC=CC=C3)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](C)NC(=O)[C@@H](C)NC(=O)[C@H](CCC(=O)N)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC4=CC=C(C=C4)O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H]([C@H](C)O)NC(=O)[C@H](CC5=CC=CC=C5)NC(=O)[C@H]([C@H](C)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)CNC(=O)[C@H](CC6=CN=CN6)N | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C148H224N40O45 | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
3283.6 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Albiglutide is an agonist of the GLP-1 (glucagon-like peptide 1) receptor and augments glucose-dependent insulin secretion. Albiglutide also slows gastric emptying., Tanzeum is an agonist of the GLP-1 receptor and augments glucose-dependent insulin secretion. Tanzeum also slows gastric emptying. | |
| Details | NIH; DailyMed. Current Medication Information for Tanzeum (Albiglutide) Injection, Powder, Lyophilized, For Solution (Updated: May 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fcad939-76e7-49cf-af94-4e6aef17901f | |
| Record name | Albiglutide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09043 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Details | NIH; DailyMed. Current Medication Information for Tanzeum (Albiglutide) Injection, Powder, Lyophilized, For Solution (Updated: May 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fcad939-76e7-49cf-af94-4e6aef17901f | |
| Record name | Albiglutide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8282 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to yellow powder | |
CAS No. |
782500-75-8 | |
| Record name | Albiglutide [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0782500758 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Albiglutide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09043 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Albiglutide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8282 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Q1: What is the mechanism of action of albiglutide?
A1: Albiglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA). [] It exerts its therapeutic effect by binding to and activating GLP-1 receptors. [, , ] This activation triggers a cascade of downstream effects, primarily in the pancreas:
- Increased Insulin Secretion: Albiglutide enhances glucose-dependent insulin secretion from pancreatic β-cells. [, , ] This means insulin release is amplified when blood glucose levels are elevated, such as after a meal.
- Decreased Glucagon Secretion: Simultaneously, albiglutide suppresses glucagon secretion from pancreatic α-cells. [, , ] Glucagon normally raises blood glucose levels; therefore, its suppression contributes to improved glycemic control.
Q2: What are the additional effects of albiglutide beyond the pancreas?
A2: In addition to its pancreatic effects, albiglutide influences other physiological processes:
- Delayed Gastric Emptying: Albiglutide slows down the rate at which food empties from the stomach into the small intestine. [, , ] This contributes to a feeling of fullness and can help regulate post-meal blood glucose levels.
- Increased Satiety: Albiglutide acts on the central nervous system to promote a sense of satiety or fullness, further contributing to its potential for weight management. [, , ]
Q3: What is the molecular structure of albiglutide?
A4: Albiglutide is a large molecule comprised of two identical chains of modified human glucagon-like peptide-1 (GLP-1) linked to a recombinant human albumin molecule. [, , ] The specific modifications within the GLP-1 chains confer resistance to DPP-4 degradation, a key factor in its extended half-life.
Q4: What are the molecular formula and weight of albiglutide?
A4: Due to the complexity of albiglutide's structure as a fusion protein, providing a precise molecular formula and weight is not straightforward. It's more relevant to consider its amino acid sequence and modifications when understanding its properties.
Q5: Is there spectroscopic data available for albiglutide?
A5: Spectroscopic data, such as that from nuclear magnetic resonance (NMR) or mass spectrometry, is crucial for characterizing protein structure. While publicly available research articles may not always provide this detailed data, it's likely utilized during the drug development process to confirm albiglutide's identity, purity, and structural integrity.
Q6: How is albiglutide absorbed and distributed in the body?
A7: Following subcutaneous administration, albiglutide is primarily absorbed via the lymphatic circulation. [] Its distribution is largely influenced by its fusion to human albumin, a protein abundant in plasma. This fusion contributes to its long half-life and allows for once-weekly dosing. [, , ]
Q7: How is albiglutide metabolized and excreted?
A8: As a large peptide, albiglutide's metabolism differs from small molecule drugs. It's likely broken down into smaller peptides and amino acids through proteolysis, a process involving enzymes. While specific details on its metabolic pathways may not be extensively published, its elimination half-life of approximately 5 days suggests a slow clearance process. [, ]
Q8: How does albiglutide affect glucose levels in patients with type 2 diabetes?
A9: Clinical trials consistently demonstrate albiglutide's efficacy in lowering both fasting plasma glucose (FPG) and postprandial plasma glucose (PPG), with HbA1c reductions ranging from -0.55% to -0.9%. [, , , , , , , , ] This glucose-lowering effect is attributed to its multi-faceted mechanism involving increased insulin secretion, decreased glucagon secretion, and delayed gastric emptying. [, , , ]
Q9: Does albiglutide cause weight loss?
A10: While albiglutide doesn't typically cause significant weight loss compared to placebo, clinical trials have shown it can lead to modest weight reductions ranging from +0.28 to -1.21 kg, depending on the comparator drug and study population. [, , , , , , , , ] Its weight management potential is attributed to its ability to increase satiety and slow gastric emptying. [, , ]
Q10: Has albiglutide been tested in preclinical models?
A11: Yes, preclinical studies in rats have demonstrated albiglutide's protective effects against ischemia/reperfusion injury, a condition that deprives the heart of oxygen. [] The study found that albiglutide significantly reduced infarct size and improved cardiac function and energetics post-injury. [] These benefits were associated with enhanced myocardial glucose uptake and a shift towards a more favorable cardiac metabolism. []
Q11: What were the main findings of the HARMONY clinical trial program?
A12: The HARMONY program encompassed eight Phase III clinical trials, representing a comprehensive evaluation of albiglutide's efficacy and safety in various patient populations with type 2 diabetes. [, , , , , , , ] These trials compared albiglutide to placebo, other GLP-1 receptor agonists, and other classes of diabetes medications, revealing key findings:
- Superior Glycemic Control: Albiglutide consistently demonstrated superior reductions in HbA1c and fasting plasma glucose compared to placebo and certain active comparators, including sitagliptin and glimepiride. [, , , , , , , ]
- Weight Management: While not as potent as some other GLP-1RAs in this regard, albiglutide demonstrated either weight neutrality or modest weight loss in some trials. [, , , , , , , ]
- Cardiovascular Safety: A key concern with diabetes medications is their potential impact on cardiovascular health. The HARMONY Outcomes trial, a major component of the program, investigated albiglutide's cardiovascular safety in patients with established cardiovascular disease. [] Notably, it demonstrated a 25% relative risk reduction in myocardial infarction (heart attack) across various infarction types. []
Q12: What is the safety profile of albiglutide?
A13: In clinical trials, albiglutide demonstrated a generally favorable safety and tolerability profile. [, , , , , , , , ] The most common adverse events were gastrointestinal in nature, primarily:
- Nausea: Experienced by a greater proportion of patients receiving albiglutide compared to placebo, but generally mild to moderate in severity. [, , ]
- Diarrhea: Similar in incidence to nausea, typically mild to moderate, and often resolving with continued treatment. [, , ]
- Injection Site Reactions: Reported in a smaller percentage of patients, typically characterized by redness or mild pain at the injection site. [, , ]
Q13: Are there any serious safety concerns associated with albiglutide?
A14: While albiglutide is generally well-tolerated, there have been rare reports of pancreatitis (inflammation of the pancreas) associated with its use. [, , ] Patients with a history of pancreatitis should avoid albiglutide. [, , ] Additionally, as with other GLP-1RAs, a potential risk for thyroid C-cell tumors has been observed in rodent studies, though it remains unclear whether this translates to humans. [] Albiglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2. []
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![Gal|A(1-3)[Neu5Ac|A(2-6)]GlcNAc-|A-pNP](/img/structure/B3029696.png)