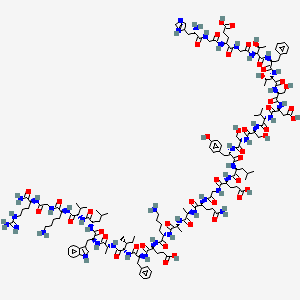
H-His-Gly-Glu-Gly-aThr-Phe-aThr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-D-Ala-D-Ala-Lys-Glu-Phe-aIle-D-Ala-Trp-Leu-Val-Lys-Gly-Arg-NH2
説明
アルビグルチドは、2型糖尿病の治療に使用されるグルカゴン様ペプチド-1受容体アゴニストです。ヨーロッパではEperzan、米国ではTanzeumという商品名で販売されています。 アルビグルチドは、ヒトグルカゴン様ペプチド-1の組換えDNAで生産されたポリペプチドアナログであり、グルコース依存性インスリン分泌の増強、不適切なグルカゴン分泌の抑制、胃の排泄遅延、食餌摂取量の減少を目的として設計されています .
作用機序
アルビグルチドは、グルカゴン様ペプチド-1受容体のアゴニストとして作用します。この受容体活性化は、膵臓のβ細胞からのグルコース依存性インスリン分泌の増加につながります。さらに、アルビグルチドはグルカゴンの分泌を抑制し、胃の排泄を遅らせ、満腹感をもたらします。 これらの複合的な作用は、血糖値の調節に役立ちます .
生化学分析
Biochemical Properties
Albiglutide acts as an agonist at the GLP-1 receptor, which makes it a type of incretin mimetic . This causes an increase in insulin secretion, predominantly in the presence of high blood glucose, and also slows down gastric emptying .
Cellular Effects
Albiglutide has a significant impact on various types of cells and cellular processes. It influences cell function by increasing insulin secretion, predominantly in the presence of high blood glucose . This can have a profound effect on cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
The molecular mechanism of action of Albiglutide involves its role as an agonist of the GLP-1 receptor . This interaction leads to an increase in insulin secretion, predominantly in the presence of high blood glucose. It also slows down gastric emptying, which can have a significant impact on the body’s metabolic processes .
Temporal Effects in Laboratory Settings
It is known that Albiglutide has a half-life of 5 (4–7) days , indicating its stability and potential for long-term effects on cellular function.
Metabolic Pathways
Albiglutide is involved in the incretin metabolic pathway, where it acts as an agonist of the GLP-1 receptor . This interaction leads to an increase in insulin secretion, predominantly in the presence of high blood glucose, and also slows down gastric emptying .
準備方法
合成経路と反応条件: アルビグルチドは、組換えDNA技術を用いて合成されます。このプロセスには、アルビグルチドポリペプチドをコードする遺伝子を適切な発現ベクターに挿入することが含まれ、その後、このベクターは大腸菌や酵母など、宿主細胞に導入されます。 宿主細胞は、アルビグルチドポリペプチドを発現させるために特定の条件下で培養され、その後、一連のクロマトグラフィー技術によって精製されます .
工業的生産方法: アルビグルチドの工業的生産は、同様の組換えDNAアプローチに従っていますが、より大規模に行われます。生産プロセスには、発酵、細胞溶解、タンパク質抽出、精製が含まれます。 最終製品は、臨床用途のために皮下注射剤に製剤化されます .
化学反応の分析
反応の種類: アルビグルチドは、主に体内でタンパク質分解によって分解されます。 ペプチドの性質により、酸化、還元、置換などの一般的な化学反応には参加しません .
一般的な試薬と条件: アルビグルチドの分解には、プロテアーゼによる酵素的切断が伴います。 これらの反応の特定の条件は生理的であり、人体内で起こります .
形成される主な生成物: アルビグルチドの分解から生成される主な生成物は、より小さいペプチド断片とアミノ酸であり、これらはさらに代謝または排泄されます .
4. 科学研究の用途
アルビグルチドは、特に医学と薬理学の分野で、いくつかの科学研究の用途があります。
医学: アルビグルチドは、2型糖尿病患者の血糖値管理に使用されます。
薬理学: アルビグルチドに関する研究には、その薬物動態と薬力学の研究、長期的な影響の理解、他のグルカゴン様ペプチド-1受容体アゴニストとの有効性の比較などが含まれます.
心臓血管研究: アルビグルチドは、2型糖尿病患者における心血管イベントのリスク軽減効果など、潜在的な心血管上の利点について調査されてきました.
科学的研究の応用
Albiglutide has several scientific research applications, particularly in the fields of medicine and pharmacology:
Medicine: Albiglutide is used to manage blood glucose levels in patients with type 2 diabetes mellitus.
Cardiovascular Research: Albiglutide has been investigated for its potential cardiovascular benefits, including its effects on reducing the risk of cardiovascular events in patients with type 2 diabetes.
類似化合物との比較
アルビグルチドは、グルカゴン様ペプチド-1受容体アゴニストとして知られる薬物の一種です。このクラスの類似化合物には、リラグルチド、エクセナチド、デュラグルチド、セマグルチドなどがあります。
比較:
リラグルチド: リラグルチドは、アルビグルチドと比較して半減期が短く、毎日投与する必要があるのに対し、アルビグルチドは週に1回投与されます.
エクセナチド: エクセナチドは、作用時間が短い別のグルカゴン様ペプチド-1受容体アゴニストであり、製剤に応じて1日に2回または週に1回の投与が必要です.
デュラグルチド: デュラグルチドは、アルビグルチドと同様に週に1回投与されますが、グリコシル化ヘモグロビン値の低下においてより高い有効性を示しています.
セマグルチド: セマグルチドは、アルビグルチドと比較して血糖コントロールと体重減少において優れた有効性を示しており、一部の患者にとってより強力な選択肢となります.
独自性: アルビグルチドの独自の特徴は、ヒトアルブミンと融合していることで、半減期が延長され、週に1回の投与が可能になっています。 この融合は、他のグルカゴン様ペプチド-1受容体アゴニストと比較して、免疫原性のリスクを軽減します .
特性
IUPAC Name |
(4S)-5-[[2-[[(2S,3S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-5-amino-1-[[(2R)-1-[[(2R)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3R)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[2-[[(2S)-1-amino-5-carbamimidamido-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-2-oxoethyl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]acetyl]amino]-5-oxopentanoic acid | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C148H224N40O45/c1-16-76(10)119(145(231)166-79(13)125(211)174-103(59-85-62-158-90-35-24-23-34-88(85)90)135(221)176-99(55-73(4)5)136(222)185-117(74(6)7)143(229)173-92(36-25-27-51-149)127(213)160-65-109(196)167-91(122(153)208)38-29-53-157-148(154)155)187-137(223)101(56-82-30-19-17-20-31-82)177-132(218)97(46-50-115(204)205)172-131(217)93(37-26-28-52-150)170-124(210)78(12)164-123(209)77(11)165-130(216)96(43-47-108(152)195)169-111(198)66-161-129(215)95(45-49-114(202)203)171-133(219)98(54-72(2)3)175-134(220)100(58-84-39-41-87(194)42-40-84)178-140(226)105(68-189)181-142(228)107(70-191)182-144(230)118(75(8)9)186-139(225)104(61-116(206)207)179-141(227)106(69-190)183-147(233)121(81(15)193)188-138(224)102(57-83-32-21-18-22-33-83)180-146(232)120(80(14)192)184-112(199)67-162-128(214)94(44-48-113(200)201)168-110(197)64-159-126(212)89(151)60-86-63-156-71-163-86/h17-24,30-35,39-42,62-63,71-81,89,91-107,117-121,158,189-194H,16,25-29,36-38,43-61,64-70,149-151H2,1-15H3,(H2,152,195)(H2,153,208)(H,156,163)(H,159,212)(H,160,213)(H,161,215)(H,162,214)(H,164,209)(H,165,216)(H,166,231)(H,167,196)(H,168,197)(H,169,198)(H,170,210)(H,171,219)(H,172,217)(H,173,229)(H,174,211)(H,175,220)(H,176,221)(H,177,218)(H,178,226)(H,179,227)(H,180,232)(H,181,228)(H,182,230)(H,183,233)(H,184,199)(H,185,222)(H,186,225)(H,187,223)(H,188,224)(H,200,201)(H,202,203)(H,204,205)(H,206,207)(H4,154,155,157)/t76-,77-,78-,79-,80+,81+,89+,91+,92+,93+,94+,95+,96+,97+,98+,99+,100+,101+,102+,103+,104+,105+,106+,107+,117+,118+,119+,120+,121+/m1/s1 | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JYDZPPZAYQTOIV-OTSUTHPESA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC(C)C(C(=O)NC(C)C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCCNC(=N)N)C(=O)N)NC(=O)C(CC3=CC=CC=C3)NC(=O)C(CCC(=O)O)NC(=O)C(CCCCN)NC(=O)C(C)NC(=O)C(C)NC(=O)C(CCC(=O)N)NC(=O)CNC(=O)C(CCC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(CO)NC(=O)C(CO)NC(=O)C(C(C)C)NC(=O)C(CC(=O)O)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)C(C(C)O)NC(=O)CNC(=O)C(CCC(=O)O)NC(=O)CNC(=O)C(CC6=CN=CN6)N | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC[C@@H](C)[C@@H](C(=O)N[C@H](C)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(=N)N)C(=O)N)NC(=O)[C@H](CC3=CC=CC=C3)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](C)NC(=O)[C@@H](C)NC(=O)[C@H](CCC(=O)N)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC4=CC=C(C=C4)O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H]([C@H](C)O)NC(=O)[C@H](CC5=CC=CC=C5)NC(=O)[C@H]([C@H](C)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)CNC(=O)[C@H](CC6=CN=CN6)N | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C148H224N40O45 | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
3283.6 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Albiglutide is an agonist of the GLP-1 (glucagon-like peptide 1) receptor and augments glucose-dependent insulin secretion. Albiglutide also slows gastric emptying., Tanzeum is an agonist of the GLP-1 receptor and augments glucose-dependent insulin secretion. Tanzeum also slows gastric emptying. | |
| Details | NIH; DailyMed. Current Medication Information for Tanzeum (Albiglutide) Injection, Powder, Lyophilized, For Solution (Updated: May 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fcad939-76e7-49cf-af94-4e6aef17901f | |
| Record name | Albiglutide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09043 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Details | NIH; DailyMed. Current Medication Information for Tanzeum (Albiglutide) Injection, Powder, Lyophilized, For Solution (Updated: May 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fcad939-76e7-49cf-af94-4e6aef17901f | |
| Record name | Albiglutide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8282 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to yellow powder | |
CAS No. |
782500-75-8 | |
| Record name | Albiglutide [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0782500758 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Albiglutide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09043 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Albiglutide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8282 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Q1: What is the mechanism of action of albiglutide?
A1: Albiglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA). [] It exerts its therapeutic effect by binding to and activating GLP-1 receptors. [, , ] This activation triggers a cascade of downstream effects, primarily in the pancreas:
- Increased Insulin Secretion: Albiglutide enhances glucose-dependent insulin secretion from pancreatic β-cells. [, , ] This means insulin release is amplified when blood glucose levels are elevated, such as after a meal.
- Decreased Glucagon Secretion: Simultaneously, albiglutide suppresses glucagon secretion from pancreatic α-cells. [, , ] Glucagon normally raises blood glucose levels; therefore, its suppression contributes to improved glycemic control.
Q2: What are the additional effects of albiglutide beyond the pancreas?
A2: In addition to its pancreatic effects, albiglutide influences other physiological processes:
- Delayed Gastric Emptying: Albiglutide slows down the rate at which food empties from the stomach into the small intestine. [, , ] This contributes to a feeling of fullness and can help regulate post-meal blood glucose levels.
- Increased Satiety: Albiglutide acts on the central nervous system to promote a sense of satiety or fullness, further contributing to its potential for weight management. [, , ]
Q3: What is the molecular structure of albiglutide?
A4: Albiglutide is a large molecule comprised of two identical chains of modified human glucagon-like peptide-1 (GLP-1) linked to a recombinant human albumin molecule. [, , ] The specific modifications within the GLP-1 chains confer resistance to DPP-4 degradation, a key factor in its extended half-life.
Q4: What are the molecular formula and weight of albiglutide?
A4: Due to the complexity of albiglutide's structure as a fusion protein, providing a precise molecular formula and weight is not straightforward. It's more relevant to consider its amino acid sequence and modifications when understanding its properties.
Q5: Is there spectroscopic data available for albiglutide?
A5: Spectroscopic data, such as that from nuclear magnetic resonance (NMR) or mass spectrometry, is crucial for characterizing protein structure. While publicly available research articles may not always provide this detailed data, it's likely utilized during the drug development process to confirm albiglutide's identity, purity, and structural integrity.
Q6: How is albiglutide absorbed and distributed in the body?
A7: Following subcutaneous administration, albiglutide is primarily absorbed via the lymphatic circulation. [] Its distribution is largely influenced by its fusion to human albumin, a protein abundant in plasma. This fusion contributes to its long half-life and allows for once-weekly dosing. [, , ]
Q7: How is albiglutide metabolized and excreted?
A8: As a large peptide, albiglutide's metabolism differs from small molecule drugs. It's likely broken down into smaller peptides and amino acids through proteolysis, a process involving enzymes. While specific details on its metabolic pathways may not be extensively published, its elimination half-life of approximately 5 days suggests a slow clearance process. [, ]
Q8: How does albiglutide affect glucose levels in patients with type 2 diabetes?
A9: Clinical trials consistently demonstrate albiglutide's efficacy in lowering both fasting plasma glucose (FPG) and postprandial plasma glucose (PPG), with HbA1c reductions ranging from -0.55% to -0.9%. [, , , , , , , , ] This glucose-lowering effect is attributed to its multi-faceted mechanism involving increased insulin secretion, decreased glucagon secretion, and delayed gastric emptying. [, , , ]
Q9: Does albiglutide cause weight loss?
A10: While albiglutide doesn't typically cause significant weight loss compared to placebo, clinical trials have shown it can lead to modest weight reductions ranging from +0.28 to -1.21 kg, depending on the comparator drug and study population. [, , , , , , , , ] Its weight management potential is attributed to its ability to increase satiety and slow gastric emptying. [, , ]
Q10: Has albiglutide been tested in preclinical models?
A11: Yes, preclinical studies in rats have demonstrated albiglutide's protective effects against ischemia/reperfusion injury, a condition that deprives the heart of oxygen. [] The study found that albiglutide significantly reduced infarct size and improved cardiac function and energetics post-injury. [] These benefits were associated with enhanced myocardial glucose uptake and a shift towards a more favorable cardiac metabolism. []
Q11: What were the main findings of the HARMONY clinical trial program?
A12: The HARMONY program encompassed eight Phase III clinical trials, representing a comprehensive evaluation of albiglutide's efficacy and safety in various patient populations with type 2 diabetes. [, , , , , , , ] These trials compared albiglutide to placebo, other GLP-1 receptor agonists, and other classes of diabetes medications, revealing key findings:
- Superior Glycemic Control: Albiglutide consistently demonstrated superior reductions in HbA1c and fasting plasma glucose compared to placebo and certain active comparators, including sitagliptin and glimepiride. [, , , , , , , ]
- Weight Management: While not as potent as some other GLP-1RAs in this regard, albiglutide demonstrated either weight neutrality or modest weight loss in some trials. [, , , , , , , ]
- Cardiovascular Safety: A key concern with diabetes medications is their potential impact on cardiovascular health. The HARMONY Outcomes trial, a major component of the program, investigated albiglutide's cardiovascular safety in patients with established cardiovascular disease. [] Notably, it demonstrated a 25% relative risk reduction in myocardial infarction (heart attack) across various infarction types. []
Q12: What is the safety profile of albiglutide?
A13: In clinical trials, albiglutide demonstrated a generally favorable safety and tolerability profile. [, , , , , , , , ] The most common adverse events were gastrointestinal in nature, primarily:
- Nausea: Experienced by a greater proportion of patients receiving albiglutide compared to placebo, but generally mild to moderate in severity. [, , ]
- Diarrhea: Similar in incidence to nausea, typically mild to moderate, and often resolving with continued treatment. [, , ]
- Injection Site Reactions: Reported in a smaller percentage of patients, typically characterized by redness or mild pain at the injection site. [, , ]
Q13: Are there any serious safety concerns associated with albiglutide?
A14: While albiglutide is generally well-tolerated, there have been rare reports of pancreatitis (inflammation of the pancreas) associated with its use. [, , ] Patients with a history of pancreatitis should avoid albiglutide. [, , ] Additionally, as with other GLP-1RAs, a potential risk for thyroid C-cell tumors has been observed in rodent studies, though it remains unclear whether this translates to humans. [] Albiglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![3,4-Dihydro-2H-pyrano[3,2-b]pyridin-4-ol](/img/structure/B3029695.png)
![[6]-Dehydrogingerdione](/img/structure/B3029713.png)