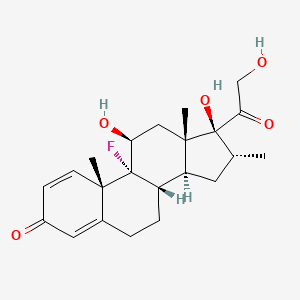
Dexamethasone
Vue d'ensemble
Description
La dexamethasone est un glucocorticoïde synthétique aux propriétés anti-inflammatoires et immunosuppressives puissantes. Elle est largement utilisée dans le traitement de diverses affections inflammatoires et auto-immunes, ainsi que dans la prise en charge de certains cancers. La this compound est connue pour sa capacité à réduire l’inflammation et à supprimer la réponse immunitaire, ce qui en fait un agent thérapeutique précieux dans de nombreuses conditions médicales .
Mécanisme D'action
La dexamethasone exerce ses effets en se liant au récepteur des glucocorticoïdes, un type de récepteur nucléaire. Lors de la liaison, le complexe this compound-récepteur se transloque dans le noyau cellulaire, où il module l’expression de gènes spécifiques impliqués dans les réponses inflammatoires et immunitaires. Cela conduit à la suppression des cytokines pro-inflammatoires et à l’inhibition de la migration des leucocytes vers les sites d’inflammation. De plus, la this compound affecte diverses voies métaboliques, y compris le métabolisme du glucose et le catabolisme des protéines .
Composés similaires :
Prednisone : Un autre glucocorticoïde ayant des propriétés anti-inflammatoires similaires mais une durée d’action plus courte.
Hydrocortisone : Un glucocorticoïde naturel ayant une puissance inférieure à celle de la this compound.
Betamethasone : Structurellement similaire à la this compound mais ayant des propriétés pharmacocinétiques légèrement différentes .
Unicité de la this compound : La this compound est unique en raison de sa forte puissance et de sa longue durée d’action. Elle est considérablement plus puissante que la prednisone et l’hydrocortisone, ce qui la rend efficace à des doses plus faibles. De plus, sa structure fluorée contribue à sa stabilité et à sa biodisponibilité accrues .
Applications De Recherche Scientifique
Dexamethasone has a wide range of scientific research applications:
Chemistry: Used as a reference compound in analytical chemistry for the development of new analytical methods.
Biology: Employed in cell culture studies to investigate the effects of glucocorticoids on cellular processes.
Medicine: Extensively used in clinical research to study its efficacy in treating inflammatory and autoimmune diseases, as well as its role in cancer therapy.
Industry: Utilized in the development of drug delivery systems and formulations to enhance the therapeutic efficacy of glucocorticoids
Analyse Biochimique
Biochemical Properties
Dexamethasone interacts with various enzymes, proteins, and other biomolecules. It is 6-hydroxylated by CYP3A4 to 6α- and 6β-hydroxythis compound . This compound is reversibly metabolized to 11-dehydrothis compound by corticosteroid 11-beta-dehydrogenase isozyme 2 and can also be converted back to this compound by Corticosteroid 11-beta-dehydrogenase isozyme 1 .
Cellular Effects
This compound has profound effects on various types of cells and cellular processes. It impacts cellular DNA, thereby changing gene transcription . It also influences cell function, including any impact on cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
This compound exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . It binds to the glucocorticoid receptor, and the drug-receptor complex translocates to the nucleus and acts as a transcription factor for target gene expression .
Temporal Effects in Laboratory Settings
The effects of this compound change over time in laboratory settings. For instance, this compound dose-dependently decreases glucocorticoid receptor levels and inhibits the growth of certain cell lines .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. For example, low-dose this compound lessens injury and enhances the recovery process in animal models of traumatic brain injury by reducing neuroinflammation and promoting neuroprotective mechanisms .
Metabolic Pathways
This compound is involved in various metabolic pathways. It is extensively metabolized to 6-hydroxythis compound and side-chain cleaved metabolites in human liver both in vitro and in vivo with CYP3A4 responsible for the formation of 6-hydroxylated products .
Transport and Distribution
This compound is transported and distributed within cells and tissues. It is a lipophilic molecule that easily penetrates the cell membrane and binds to intracellular cytoplasmic glucocorticoid receptors .
Subcellular Localization
This compound and its receptor complex can translocate to the nucleus, affecting gene transcription . This subcellular localization is crucial for its activity or function .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La dexamethasone est synthétisée par un processus en plusieurs étapes à partir de précurseurs stéroïdiens plus simples. Une méthode courante implique l’utilisation de la prednisolone comme matière première. La synthèse comprend des étapes telles que la fluoration, l’hydroxylation et la méthylation pour introduire les groupes fonctionnels nécessaires. Les conditions de réaction impliquent généralement l’utilisation de réactifs tels que le fluor gazeux, le peroxyde d’hydrogène et divers catalyseurs pour obtenir les transformations souhaitées .
Méthodes de production industrielle : La production industrielle de this compound implique une synthèse chimique à grande échelle utilisant des conditions de réaction optimisées pour garantir un rendement et une pureté élevés. Le processus comprend des étapes de purification rigoureuses telles que la recristallisation et la chromatographie pour obtenir le produit final. La production est réalisée sous des mesures de contrôle de qualité strictes pour répondre aux normes pharmaceutiques .
Analyse Des Réactions Chimiques
Types de réactions : La dexamethasone subit diverses réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former différents dérivés, qui peuvent avoir des propriétés pharmacologiques modifiées.
Réduction : Les réactions de réduction peuvent modifier les groupes cétones de la this compound, conduisant à différents analogues.
Substitution : L’halogénation et d’autres réactions de substitution peuvent introduire de nouveaux groupes fonctionnels dans la molécule de this compound
Réactifs et conditions courants :
Oxydation : Peroxyde d’hydrogène, permanganate de potassium.
Réduction : Borohydrure de sodium, hydrure de lithium et d’aluminium.
Substitution : Agents halogénants comme le chlore ou le brome, divers catalyseurs
Produits principaux : Les principaux produits formés à partir de ces réactions comprennent divers dérivés de la this compound ayant des applications thérapeutiques potentielles. Ces dérivés peuvent présenter des propriétés pharmacocinétiques et pharmacodynamiques différentes par rapport au composé parent .
4. Applications de la recherche scientifique
La this compound a un large éventail d’applications dans la recherche scientifique :
Chimie : Utilisée comme composé de référence en chimie analytique pour le développement de nouvelles méthodes analytiques.
Biologie : Employée dans des études de culture cellulaire pour étudier les effets des glucocorticoïdes sur les processus cellulaires.
Médecine : Largement utilisée dans la recherche clinique pour étudier son efficacité dans le traitement des maladies inflammatoires et auto-immunes, ainsi que son rôle dans la thérapie anticancéreuse.
Industrie : Utilisée dans le développement de systèmes d’administration de médicaments et de formulations pour améliorer l’efficacité thérapeutique des glucocorticoïdes
Comparaison Avec Des Composés Similaires
Prednisone: Another glucocorticoid with similar anti-inflammatory properties but shorter duration of action.
Hydrocortisone: A naturally occurring glucocorticoid with less potency compared to dexamethasone.
Betamethasone: Structurally similar to this compound but with slightly different pharmacokinetic properties .
Uniqueness of this compound: this compound is unique due to its high potency and long duration of action. It is significantly more potent than prednisone and hydrocortisone, making it effective at lower doses. Additionally, its fluorinated structure contributes to its enhanced stability and bioavailability .
Propriétés
IUPAC Name |
(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UREBDLICKHMUKA-CXSFZGCWSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CO)O)C)O)F)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CO)O)C)O)F)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H29FO5 | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3020384 | |
| Record name | Dexamethasone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020384 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
392.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Dexamethazone is an odorless white to off-white crystalline powder with a slightly bitter taste. (NTP, 1992), Solid | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
less than 1 mg/mL at 77 °F (NTP, 1992), Crystals; sol in water; max absorption (ethanol): 238-239 nm (e= 14,000); specific optical rotation: +57 deg/D (water); mp 233-235 °C; specific optical rotation: +74 +- 4 deg at 25 °C/D (water- and alc-free basis, 10 mg/mL) /21-phosphate disodium salt of dexamethasone/, ODORLESS OR HAS SLIGHT ODOR OF ALCOHOL; WHITE, OR SLIGHTLY YELLOW, CRYSTALLINE POWDER; 1 G DISSOLVES IN ABOUT 2 ML OF WATER; INSOL IN DIOXANE; SLIGHTLY SOL IN ALC; INSOL IN ETHER & CHLOROFORM; VERY HYGROSCOPIC /DEXAMETHASONE SODIUM PHOSPHATE/, Solubility in water (25 °C): 10 mg/100 mL; sol in acetone, ethanol, chloroform, In water, 89.0 mg/L at 25 °C, 5.05e-02 g/L | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels., Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA, and stimulate transcription of mRNA and subsequent protein synthesis of enzymes ultimately responsible for anti-inflammatory effects of topical application of corticosteroids to the eye. In high concentrations which may be achieved after topical application, corticosteroids may exert direct membrane effects. Corticosteroids decrease cellular and fibrinous exudation and tissue infiltration, inhibit fibroblastic and collagen-forming activity, retard epithelial regeneration, diminish postinflammatory neovascularization and reduce toward normal levels the excessive permeability of inflamed capillaries. /Corticosteroids (Otic)/, Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/, Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA (chromatin), and stimulate transcription of messenger RNA (mRNA) and subsequent protein synthesis of various inhibitory enzymes responsible for the anti-inflammatory effects of topical corticosteroids. These anti-inflammatory effects include inhibition of early processes such as edema, fibrin deposition, capillary dilatation, movement of phagocttes into the area, and phagocytic activities. Later processes, such as capillary production, collagen deposition, and keloid formation also are inhibited by corticosteroids. The overall actions of topical corticosteroids are catabolic. /Corticosteroids (topical)/ | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from ether, WHITE TO PRACTICALLY WHITE CRYSTALLINE POWDER | |
CAS No. |
50-02-2, 23495-06-9 | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, labeled with tritium, (11β,16α)- | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=23495-06-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexamethasone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-02-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexamethasone [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050022 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (3H)-Dexamethasone | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023495069 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | dexamethasone | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=34521 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11.beta.,16.alpha.)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Dexamethasone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020384 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Dexamethasone | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.004 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DEXAMETHASONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7S5I7G3JQL | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
504 to 507 °F (NTP, 1992), 260-264, 262-264 °C, 262 °C | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![5H-Dibenzo[a,d]cyclohepten-5-one O-[2-(methylamino)ethyl]oxime monohydrochloride](/img/structure/B1670265.png)
