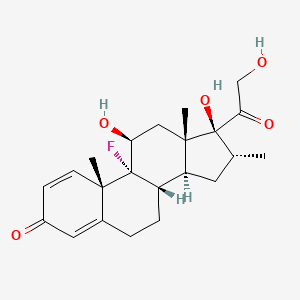
Dexametasona
Descripción general
Descripción
La dexametasona es un glucocorticoide sintético con potentes propiedades antiinflamatorias e inmunosupresoras. Es ampliamente utilizada en el tratamiento de diversas afecciones inflamatorias y autoinmunes, así como en el manejo de ciertos cánceres. La this compound es conocida por su capacidad para reducir la inflamación y suprimir la respuesta inmunitaria, convirtiéndola en un valioso agente terapéutico en numerosas afecciones médicas .
Mecanismo De Acción
La dexametasona ejerce sus efectos uniéndose al receptor de glucocorticoides, un tipo de receptor nuclear. Tras la unión, el complejo this compound-receptor se transloca al núcleo celular, donde modula la expresión de genes específicos implicados en las respuestas inflamatorias e inmunitarias. Esto conduce a la supresión de citoquinas proinflamatorias y a la inhibición de la migración de leucocitos a los sitios de inflamación. Además, la this compound afecta diversas vías metabólicas, incluido el metabolismo de la glucosa y el catabolismo de las proteínas .
Compuestos Similares:
Prednisona: Otro glucocorticoide con propiedades antiinflamatorias similares, pero con una duración de acción más corta.
Hidrocortisona: Un glucocorticoide natural con menos potencia en comparación con la this compound.
Betametasona: Estructuralmente similar a la this compound, pero con propiedades farmacocinéticas ligeramente diferentes .
Singularidad de la this compound: La this compound es única debido a su alta potencia y larga duración de acción. Es significativamente más potente que la prednisona y la hidrocortisona, lo que la hace eficaz a dosis más bajas. Además, su estructura fluorada contribuye a su mayor estabilidad y biodisponibilidad .
Aplicaciones Científicas De Investigación
La dexametasona tiene una amplia gama de aplicaciones de investigación científica:
Química: Utilizada como compuesto de referencia en química analítica para el desarrollo de nuevos métodos analíticos.
Biología: Empleada en estudios de cultivo celular para investigar los efectos de los glucocorticoides en los procesos celulares.
Medicina: Ampliamente utilizada en la investigación clínica para estudiar su eficacia en el tratamiento de enfermedades inflamatorias y autoinmunes, así como su papel en la terapia del cáncer.
Industria: Utilizada en el desarrollo de sistemas de administración de fármacos y formulaciones para mejorar la eficacia terapéutica de los glucocorticoides
Análisis Bioquímico
Biochemical Properties
Dexamethasone interacts with various enzymes, proteins, and other biomolecules. It is 6-hydroxylated by CYP3A4 to 6α- and 6β-hydroxydexamethasone . Dexamethasone is reversibly metabolized to 11-dehydrodexamethasone by corticosteroid 11-beta-dehydrogenase isozyme 2 and can also be converted back to dexamethasone by Corticosteroid 11-beta-dehydrogenase isozyme 1 .
Cellular Effects
Dexamethasone has profound effects on various types of cells and cellular processes. It impacts cellular DNA, thereby changing gene transcription . It also influences cell function, including any impact on cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
Dexamethasone exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . It binds to the glucocorticoid receptor, and the drug-receptor complex translocates to the nucleus and acts as a transcription factor for target gene expression .
Temporal Effects in Laboratory Settings
The effects of dexamethasone change over time in laboratory settings. For instance, dexamethasone dose-dependently decreases glucocorticoid receptor levels and inhibits the growth of certain cell lines .
Dosage Effects in Animal Models
The effects of dexamethasone vary with different dosages in animal models. For example, low-dose dexamethasone lessens injury and enhances the recovery process in animal models of traumatic brain injury by reducing neuroinflammation and promoting neuroprotective mechanisms .
Metabolic Pathways
Dexamethasone is involved in various metabolic pathways. It is extensively metabolized to 6-hydroxydexamethasone and side-chain cleaved metabolites in human liver both in vitro and in vivo with CYP3A4 responsible for the formation of 6-hydroxylated products .
Transport and Distribution
Dexamethasone is transported and distributed within cells and tissues. It is a lipophilic molecule that easily penetrates the cell membrane and binds to intracellular cytoplasmic glucocorticoid receptors .
Subcellular Localization
Dexamethasone and its receptor complex can translocate to the nucleus, affecting gene transcription . This subcellular localization is crucial for its activity or function .
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción: La dexametasona se sintetiza a través de un proceso de varios pasos que comienza con precursores esteroides más simples. Un método común implica el uso de prednisolona como material de partida. La síntesis incluye pasos como la fluoración, la hidroxilación y la metilación para introducir los grupos funcionales necesarios. Las condiciones de reacción suelen implicar el uso de reactivos como gas flúor, peróxido de hidrógeno y varios catalizadores para lograr las transformaciones deseadas .
Métodos de Producción Industrial: La producción industrial de this compound implica síntesis química a gran escala utilizando condiciones de reacción optimizadas para garantizar un alto rendimiento y pureza. El proceso incluye rigurosos pasos de purificación como la recristalización y la cromatografía para obtener el producto final. La producción se lleva a cabo bajo estrictas medidas de control de calidad para cumplir con los estándares farmacéuticos .
Análisis De Reacciones Químicas
Tipos de Reacciones: La dexametasona experimenta diversas reacciones químicas, que incluyen:
Oxidación: La this compound puede oxidarse para formar diferentes derivados, que pueden tener propiedades farmacológicas alteradas.
Reducción: Las reacciones de reducción pueden modificar los grupos cetónicos en la this compound, dando lugar a diferentes análogos.
Sustitución: La halogenación y otras reacciones de sustitución pueden introducir nuevos grupos funcionales en la molécula de this compound
Reactivos y Condiciones Comunes:
Oxidación: Peróxido de hidrógeno, permanganato de potasio.
Reducción: Borohidruro de sodio, hidruro de aluminio y litio.
Sustitución: Agentes halogenantes como cloro o bromo, varios catalizadores
Productos Principales: Los principales productos formados a partir de estas reacciones incluyen varios derivados de la this compound con potenciales aplicaciones terapéuticas. Estos derivados pueden exhibir diferentes propiedades farmacocinéticas y farmacodinámicas en comparación con el compuesto original .
Comparación Con Compuestos Similares
Prednisone: Another glucocorticoid with similar anti-inflammatory properties but shorter duration of action.
Hydrocortisone: A naturally occurring glucocorticoid with less potency compared to dexamethasone.
Betamethasone: Structurally similar to dexamethasone but with slightly different pharmacokinetic properties .
Uniqueness of Dexamethasone: Dexamethasone is unique due to its high potency and long duration of action. It is significantly more potent than prednisone and hydrocortisone, making it effective at lower doses. Additionally, its fluorinated structure contributes to its enhanced stability and bioavailability .
Propiedades
IUPAC Name |
(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UREBDLICKHMUKA-CXSFZGCWSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CO)O)C)O)F)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CO)O)C)O)F)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H29FO5 | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3020384 | |
| Record name | Dexamethasone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020384 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
392.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Dexamethazone is an odorless white to off-white crystalline powder with a slightly bitter taste. (NTP, 1992), Solid | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
less than 1 mg/mL at 77 °F (NTP, 1992), Crystals; sol in water; max absorption (ethanol): 238-239 nm (e= 14,000); specific optical rotation: +57 deg/D (water); mp 233-235 °C; specific optical rotation: +74 +- 4 deg at 25 °C/D (water- and alc-free basis, 10 mg/mL) /21-phosphate disodium salt of dexamethasone/, ODORLESS OR HAS SLIGHT ODOR OF ALCOHOL; WHITE, OR SLIGHTLY YELLOW, CRYSTALLINE POWDER; 1 G DISSOLVES IN ABOUT 2 ML OF WATER; INSOL IN DIOXANE; SLIGHTLY SOL IN ALC; INSOL IN ETHER & CHLOROFORM; VERY HYGROSCOPIC /DEXAMETHASONE SODIUM PHOSPHATE/, Solubility in water (25 °C): 10 mg/100 mL; sol in acetone, ethanol, chloroform, In water, 89.0 mg/L at 25 °C, 5.05e-02 g/L | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels., Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA, and stimulate transcription of mRNA and subsequent protein synthesis of enzymes ultimately responsible for anti-inflammatory effects of topical application of corticosteroids to the eye. In high concentrations which may be achieved after topical application, corticosteroids may exert direct membrane effects. Corticosteroids decrease cellular and fibrinous exudation and tissue infiltration, inhibit fibroblastic and collagen-forming activity, retard epithelial regeneration, diminish postinflammatory neovascularization and reduce toward normal levels the excessive permeability of inflamed capillaries. /Corticosteroids (Otic)/, Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/, Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA (chromatin), and stimulate transcription of messenger RNA (mRNA) and subsequent protein synthesis of various inhibitory enzymes responsible for the anti-inflammatory effects of topical corticosteroids. These anti-inflammatory effects include inhibition of early processes such as edema, fibrin deposition, capillary dilatation, movement of phagocttes into the area, and phagocytic activities. Later processes, such as capillary production, collagen deposition, and keloid formation also are inhibited by corticosteroids. The overall actions of topical corticosteroids are catabolic. /Corticosteroids (topical)/ | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from ether, WHITE TO PRACTICALLY WHITE CRYSTALLINE POWDER | |
CAS No. |
50-02-2, 23495-06-9 | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, labeled with tritium, (11β,16α)- | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=23495-06-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexamethasone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-02-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexamethasone [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050022 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (3H)-Dexamethasone | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023495069 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | dexamethasone | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=34521 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11.beta.,16.alpha.)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Dexamethasone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020384 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Dexamethasone | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.004 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DEXAMETHASONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7S5I7G3JQL | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
504 to 507 °F (NTP, 1992), 260-264, 262-264 °C, 262 °C | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![5H-Dibenzo[a,d]cyclohepten-5-one O-[2-(methylamino)ethyl]oxime monohydrochloride](/img/structure/B1670265.png)
