Dexamethasone
概要
説明
デキサメタゾンは、強力な抗炎症作用と免疫抑制作用を有する合成グルココルチコイドです。さまざまな炎症性および自己免疫性疾患の治療、ならびに特定の癌の管理に広く使用されています。 デキサメタゾンは、炎症を軽減し、免疫応答を抑制する能力で知られており、多くの医学的状態における貴重な治療薬となっています .
2. 製法
合成経路と反応条件: デキサメタゾンは、より単純なステロイド前駆体から出発する多段階プロセスによって合成されます。一般的な方法の1つは、プレドニゾロンを原料とする方法です。合成には、必要な官能基を導入するためのフッ素化、水酸化、メチル化などのステップが含まれます。 反応条件は通常、フッ素ガス、過酸化水素、さまざまな触媒などの試薬の使用を含み、目的の変換を実現します .
工業生産方法: デキサメタゾンの工業生産は、高収率と高純度を確保するための最適化された反応条件を使用した大規模な化学合成で行われます。プロセスには、最終製品を得るための再結晶およびクロマトグラフィーなどの厳格な精製手順が含まれます。 生産は、医薬品規格を満たすために、厳格な品質管理の下で行われています .
作用機序
デキサメタゾンは、核受容体の一種であるグルココルチコイド受容体に結合することで作用を発揮します。結合すると、デキサメタゾン受容体複合体は細胞核に移行し、炎症および免疫応答に関与する特定の遺伝子の発現を調節します。これにより、プロ炎症性サイトカインの抑制と、炎症部位への白血球遊走の阻害が起こります。 さらに、デキサメタゾンは、グルコース代謝やタンパク質異化作用などのさまざまな代謝経路に影響を与えます .
類似化合物:
プレドニゾン: 抗炎症作用が似ていますが、作用時間が短い別のグルココルチコイドです。
ヒドロコルチゾン: デキサメタゾンと比較して効力が低い天然のグルココルチコイドです。
ベタメタゾン: 構造的にはデキサメタゾンに似ていますが、薬物動態がわずかに異なります .
デキサメタゾンの独自性: デキサメタゾンは、その高い効力と長い作用時間で際立っています。プレドニゾンやヒドロコルチゾンよりもはるかに効力が強く、低用量でも効果を発揮します。 さらに、フッ素化された構造は、その安定性と生物学的利用能の向上に貢献しています .
生化学分析
Biochemical Properties
Dexamethasone interacts with various enzymes, proteins, and other biomolecules. It is 6-hydroxylated by CYP3A4 to 6α- and 6β-hydroxythis compound . This compound is reversibly metabolized to 11-dehydrothis compound by corticosteroid 11-beta-dehydrogenase isozyme 2 and can also be converted back to this compound by Corticosteroid 11-beta-dehydrogenase isozyme 1 .
Cellular Effects
This compound has profound effects on various types of cells and cellular processes. It impacts cellular DNA, thereby changing gene transcription . It also influences cell function, including any impact on cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
This compound exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . It binds to the glucocorticoid receptor, and the drug-receptor complex translocates to the nucleus and acts as a transcription factor for target gene expression .
Temporal Effects in Laboratory Settings
The effects of this compound change over time in laboratory settings. For instance, this compound dose-dependently decreases glucocorticoid receptor levels and inhibits the growth of certain cell lines .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. For example, low-dose this compound lessens injury and enhances the recovery process in animal models of traumatic brain injury by reducing neuroinflammation and promoting neuroprotective mechanisms .
Metabolic Pathways
This compound is involved in various metabolic pathways. It is extensively metabolized to 6-hydroxythis compound and side-chain cleaved metabolites in human liver both in vitro and in vivo with CYP3A4 responsible for the formation of 6-hydroxylated products .
Transport and Distribution
This compound is transported and distributed within cells and tissues. It is a lipophilic molecule that easily penetrates the cell membrane and binds to intracellular cytoplasmic glucocorticoid receptors .
Subcellular Localization
This compound and its receptor complex can translocate to the nucleus, affecting gene transcription . This subcellular localization is crucial for its activity or function .
準備方法
Synthetic Routes and Reaction Conditions: Dexamethasone is synthesized through a multi-step process starting from simpler steroid precursors. One common method involves the use of prednisolone as a starting material. The synthesis includes steps such as fluorination, hydroxylation, and methylation to introduce the necessary functional groups. The reaction conditions typically involve the use of reagents like fluorine gas, hydrogen peroxide, and various catalysts to achieve the desired transformations .
Industrial Production Methods: Industrial production of this compound involves large-scale chemical synthesis using optimized reaction conditions to ensure high yield and purity. The process includes rigorous purification steps such as recrystallization and chromatography to obtain the final product. The production is carried out under strict quality control measures to meet pharmaceutical standards .
化学反応の分析
反応の種類: デキサメタゾンは、以下を含むさまざまな化学反応を起こします。
酸化: デキサメタゾンは酸化されて、薬理作用が異なるさまざまな誘導体に変換される可能性があります。
還元: 還元反応は、デキサメタゾンのケトン基を修飾し、異なる類似体をもたらす可能性があります。
一般的な試薬と条件:
酸化: 過酸化水素、過マンガン酸カリウム。
還元: 水素化ホウ素ナトリウム、水素化アルミニウムリチウム。
主な生成物: これらの反応から生成される主な生成物には、潜在的な治療用途を持つさまざまなデキサメタゾン誘導体が含まれます。 これらの誘導体は、親化合物と比較して、異なる薬物動態および薬力学を示す可能性があります .
4. 科学研究への応用
デキサメタゾンは、幅広い科学研究への応用があります。
化学: 新しい分析方法の開発のための分析化学における参照化合物として使用されます。
生物学: グルココルチコイドが細胞プロセスに与える影響を調査するための細胞培養研究で使用されます。
医学: 炎症性および自己免疫性疾患の治療における有効性、および癌治療における役割を調査するために、臨床研究で広く使用されています。
科学的研究の応用
Dexamethasone has a wide range of scientific research applications:
Chemistry: Used as a reference compound in analytical chemistry for the development of new analytical methods.
Biology: Employed in cell culture studies to investigate the effects of glucocorticoids on cellular processes.
Medicine: Extensively used in clinical research to study its efficacy in treating inflammatory and autoimmune diseases, as well as its role in cancer therapy.
Industry: Utilized in the development of drug delivery systems and formulations to enhance the therapeutic efficacy of glucocorticoids
類似化合物との比較
Prednisone: Another glucocorticoid with similar anti-inflammatory properties but shorter duration of action.
Hydrocortisone: A naturally occurring glucocorticoid with less potency compared to dexamethasone.
Betamethasone: Structurally similar to this compound but with slightly different pharmacokinetic properties .
Uniqueness of this compound: this compound is unique due to its high potency and long duration of action. It is significantly more potent than prednisone and hydrocortisone, making it effective at lower doses. Additionally, its fluorinated structure contributes to its enhanced stability and bioavailability .
特性
IUPAC Name |
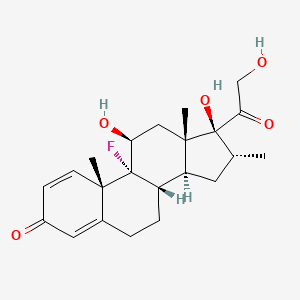
(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UREBDLICKHMUKA-CXSFZGCWSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CO)O)C)O)F)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CO)O)C)O)F)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H29FO5 | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3020384 | |
| Record name | Dexamethasone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020384 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
392.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Dexamethazone is an odorless white to off-white crystalline powder with a slightly bitter taste. (NTP, 1992), Solid | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
less than 1 mg/mL at 77 °F (NTP, 1992), Crystals; sol in water; max absorption (ethanol): 238-239 nm (e= 14,000); specific optical rotation: +57 deg/D (water); mp 233-235 °C; specific optical rotation: +74 +- 4 deg at 25 °C/D (water- and alc-free basis, 10 mg/mL) /21-phosphate disodium salt of dexamethasone/, ODORLESS OR HAS SLIGHT ODOR OF ALCOHOL; WHITE, OR SLIGHTLY YELLOW, CRYSTALLINE POWDER; 1 G DISSOLVES IN ABOUT 2 ML OF WATER; INSOL IN DIOXANE; SLIGHTLY SOL IN ALC; INSOL IN ETHER & CHLOROFORM; VERY HYGROSCOPIC /DEXAMETHASONE SODIUM PHOSPHATE/, Solubility in water (25 °C): 10 mg/100 mL; sol in acetone, ethanol, chloroform, In water, 89.0 mg/L at 25 °C, 5.05e-02 g/L | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels., Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA, and stimulate transcription of mRNA and subsequent protein synthesis of enzymes ultimately responsible for anti-inflammatory effects of topical application of corticosteroids to the eye. In high concentrations which may be achieved after topical application, corticosteroids may exert direct membrane effects. Corticosteroids decrease cellular and fibrinous exudation and tissue infiltration, inhibit fibroblastic and collagen-forming activity, retard epithelial regeneration, diminish postinflammatory neovascularization and reduce toward normal levels the excessive permeability of inflamed capillaries. /Corticosteroids (Otic)/, Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/, Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA (chromatin), and stimulate transcription of messenger RNA (mRNA) and subsequent protein synthesis of various inhibitory enzymes responsible for the anti-inflammatory effects of topical corticosteroids. These anti-inflammatory effects include inhibition of early processes such as edema, fibrin deposition, capillary dilatation, movement of phagocttes into the area, and phagocytic activities. Later processes, such as capillary production, collagen deposition, and keloid formation also are inhibited by corticosteroids. The overall actions of topical corticosteroids are catabolic. /Corticosteroids (topical)/ | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from ether, WHITE TO PRACTICALLY WHITE CRYSTALLINE POWDER | |
CAS No. |
50-02-2, 23495-06-9 | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, labeled with tritium, (11β,16α)- | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=23495-06-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexamethasone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-02-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexamethasone [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050022 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (3H)-Dexamethasone | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023495069 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | dexamethasone | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=34521 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11.beta.,16.alpha.)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Dexamethasone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020384 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Dexamethasone | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.004 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DEXAMETHASONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7S5I7G3JQL | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
504 to 507 °F (NTP, 1992), 260-264, 262-264 °C, 262 °C | |
| Record name | DEXAMETHAZONE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20100 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Dexamethasone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01234 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DEXAMETHASONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3053 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Dexamethasone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015364 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。