Clindamycin
Übersicht
Beschreibung
Clindamycin is a lincosamide antibiotic used to treat a variety of bacterial infections. It is particularly effective against anaerobic bacteria and certain gram-positive bacteria, including staphylococci and streptococci. This compound is commonly used to treat infections such as osteomyelitis, pelvic inflammatory disease, strep throat, pneumonia, and skin infections . It is available in various forms, including oral capsules, topical creams, and intravenous solutions .
Wirkmechanismus
Target of Action
Clindamycin, a lincosamide antibiotic, primarily targets the 50s ribosomal subunit of bacteria . This subunit plays a crucial role in protein synthesis, making it an effective target for this compound .
Mode of Action
This compound operates by inhibiting bacterial protein synthesis . It achieves this by binding to the 50s subunit of the bacterial ribosome, thus preventing the elongation of the peptide chain during translation . This disruption of protein synthesis interferes with the transpeptidation reaction, thereby inhibiting early chain elongation .
Biochemical Pathways
By disrupting bacterial protein synthesis, this compound causes changes in the cell wall surface, which decreases adherence of bacteria to host cells and increases intracellular killing of organisms . The drug also exerts an extended postantibiotic effect against some strains of bacteria, which may be attributed to the persistence of the drug at the ribosomal binding site .
Pharmacokinetics
This compound exhibits high bioavailability, with approximately 90% of an oral dose rapidly absorbed from the gastrointestinal tract . Peak serum concentrations are attained within 45 minutes . This compound is metabolized in the liver and excreted via the bile duct and kidneys .
Result of Action
The primary result of this compound’s action is the effective treatment of a variety of serious infections due to susceptible microorganisms . These include anaerobic bacteria as well as gram-positive cocci and bacilli . This compound is also used for antimicrobial prophylaxis against Viridans group streptococcal infections in susceptible patients undergoing oral, dental, or upper respiratory surgery .
Action Environment
The action of this compound can be influenced by environmental factors such as the presence of other drugs. For instance, chloramphenicol and macrolides such as erythromycin, clarithromycin, and azithromycin also act at the 50s ribosomal subunit and may compete for binding at this site . Furthermore, the efficacy of this compound can be affected by the disease state and the patient’s immune response .
Wissenschaftliche Forschungsanwendungen
Clindamycin hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Es wird als Modellverbindung bei der Untersuchung der Antibiotikasynthese und -modifikation verwendet.
Biologie: this compound wird verwendet, um die bakterielle Proteinsynthese und Resistenzmechanismen zu untersuchen.
Medizin: Es wird häufig zur Behandlung bakterieller Infektionen eingesetzt, einschließlich derer, die durch Methicillin-resistente Staphylococcus aureus (MRSA) und anaerobe Bakterien verursacht werden
5. Wirkmechanismus
This compound wirkt, indem es an die 50S-ribosomale Untereinheit von Bakterien bindet und so die Proteinsynthese hemmt. Diese Wirkung verhindert die Elongation der Peptidkette während der Translation und stoppt so effektiv das Bakterienwachstum . This compound zielt auf das bakterielle Ribosom ab, stört die Transpeptidierungsreaktion und hemmt die frühe Kettenelongation .
Biochemische Analyse
Biochemical Properties
Clindamycin works primarily by binding to the 50S ribosomal subunit of bacteria . This agent disrupts protein synthesis by interfering with the transpeptidation reaction, which thereby inhibits early chain elongation . By disrupting bacterial protein synthesis, this compound causes changes in the cell wall surface, which decreases adherence of bacteria to host cells and increases intracellular killing of organisms .
Cellular Effects
This compound achieves high intracellular levels in phagocytic cells . By disrupting bacterial protein synthesis, this compound causes changes in the cell wall surface, which decreases adherence of bacteria to host cells and increases intracellular killing of organisms .
Molecular Mechanism
This compound inhibits bacterial protein synthesis by binding to 23S RNA of the 50S subunit of the bacterial ribosome . It impedes both the assembly of the ribosome and the translation process . The molecular mechanism through which this occurs is thought to be due to this compound’s three-dimensional structure, which closely resembles the 3’-ends of L-Pro-Met-tRNA and deacylated-tRNA during the peptide elongation cycle .
Temporal Effects in Laboratory Settings
This compound exerts an extended postantibiotic effect against some strains of bacteria, which may be attributed to persistence of the drug at the ribosomal binding site .
Dosage Effects in Animal Models
In veterinary medicine, this compound is used at a dosage of 10-15 mg/kg, administered orally or intravenously every 12-24 hours . There are rarely reported cases of overdosage since this compound is well tolerated even at high dosages .
Metabolic Pathways
This compound undergoes hepatic metabolism mediated primarily by CYP3A4 and, to a lesser extent, CYP3A5 . Two inactive metabolites have been identified - an oxidative metabolite, this compound sulfoxide, and an N-demethylated metabolite, N-desmethylthis compound .
Transport and Distribution
This compound is widely distributed in the body, including into bone, but does not distribute into cerebrospinal fluid . The volume of distribution has been variably estimated between 43-74 L .
Subcellular Localization
The primary targets of this compound, the 50S ribosomal subunits, are located in the cytoplasm of the bacterial cell . Therefore, the subcellular localization of this compound would be within the bacterial cytoplasm where it can exert its effects .
Vorbereitungsmethoden
Clindamycin is synthesized from lincomycin, a naturally occurring antibiotic. The synthesis involves the chlorination of lincomycin to replace the hydroxyl group at position 7 with a chlorine atom . The process includes several steps:
Silicon Protecting Group Application: Lincomycin is first protected using a silicon group.
Selective Deprotection: The protected lincomycin undergoes selective deprotection.
Mitsunobu Substitution Reaction: The deprotected lincomycin is subjected to a Mitsunobu substitution reaction.
Hydrolysis Reaction: The product is then hydrolyzed to obtain 7-epime lincomycin.
Chlorination Reaction: Finally, the 7-epime lincomycin is chlorinated to produce this compound.
Industrial production of this compound hydrochloride involves chlorination, hydrolysis, extraction, and concentration steps to obtain the free alkali form, followed by salt formation and dealcoholation to yield this compound hydrochloride .
Analyse Chemischer Reaktionen
Clindamycin unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um Sulfoxid- und N-demethylierte Metaboliten zu bilden.
Reduktion: Reduktionsreaktionen sind weniger häufig, können aber unter bestimmten Bedingungen auftreten.
Substitution: Die Mitsunobu-Substitutionsreaktion ist ein wichtiger Schritt in seiner Synthese.
Häufig verwendete Reagenzien in diesen Reaktionen sind Chlorierungsmittel, Oxidationsmittel und Reduktionsmittel. Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, sind this compound-Hydrochlorid und seine Metaboliten .
Vergleich Mit ähnlichen Verbindungen
Clindamycin wird häufig mit anderen Antibiotika verglichen, wie z. B.:
Lincomycin: this compound ist ein chloriertes Derivat von Lincomycin mit verbesserten Eigenschaften.
Amoxicillin: Ein Penicillin-Antibiotikum, das bei ähnlichen Infektionen eingesetzt wird, jedoch einen anderen Wirkmechanismus hat.
Doxycyclin: Ein Tetracyclin-Antibiotikum mit einem breiteren Wirkungsspektrum
This compound ist einzigartig aufgrund seiner hohen Wirksamkeit gegen anaerobe Bakterien und seiner Fähigkeit, Knochen und Abszesse zu durchdringen, was es besonders nützlich für die Behandlung von Osteomyelitis und anderen tief liegenden Infektionen macht .
Eigenschaften
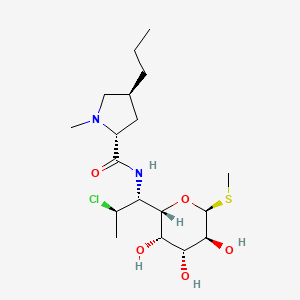
IUPAC Name |
(2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KDLRVYVGXIQJDK-AWPVFWJPSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCC1CC(N(C1)C)C(=O)NC(C2C(C(C(C(O2)SC)O)O)O)C(C)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCC[C@@H]1C[C@H](N(C1)C)C(=O)N[C@@H]([C@@H]2[C@@H]([C@@H]([C@H]([C@H](O2)SC)O)O)O)[C@H](C)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H33ClN2O5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
21462-39-5 (mono-hydrochloride), 58207-19-5 (mono-HCl, mono-hydrate) | |
| Record name | Clindamycin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0018323449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID2022836 | |
| Record name | Clindamycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
425.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Clindamycin may be bacteriostatic or bactericidal in action, depending on the concentration of the drug attained at the site of infection and the susceptibility of the infecting organism. Clindamycin palmitate hydrochloride and clindamycin phosphate are inactive until hydrolyzed to free clindamycin. This hydrolysis occurs rapidly in vivo. Clindamycin appears to inhibit protein synthesis in susceptible organisms by binding to 50S ribosomal subunits; the primary effect is inhibition of peptide bond formation. The site of action appears to be the same as that of erythromycin, chloramphenicol, and lincomycin., Clindamycin binds exclusively to the 50S subunit of bacterial ribosomes and suppresses protein synthesis., ... Clindamycin is not a substrate for macrolide efflux pumps, and strains that are resistant to macrolides by this mechanism are susceptible to clindamycin. | |
| Record name | CLINDAMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3037 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow, amorphous solid | |
CAS No. |
18323-44-9 | |
| Record name | Clindamycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=18323-44-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clindamycin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0018323449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clindamycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clindamycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.038.357 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLINDAMYCIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3U02EL437C | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLINDAMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3037 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Clindamycin exert its antibacterial effect?
A1: this compound inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit, specifically at the center of the peptidyl transferase center. [, , ] This binding prevents peptide bond formation and disrupts the translocation process, ultimately halting protein synthesis and leading to bacterial growth inhibition or death. [, ]
Q2: Does this compound possess any anti-inflammatory properties?
A3: Yes, in addition to its antibacterial action, this compound also exhibits anti-inflammatory effects. [] It has been shown to reduce inflammation associated with acne by influencing inflammatory pathways and potentially inhibiting neutrophil chemotaxis. []
Q3: What is the molecular formula and weight of this compound?
A4: The molecular formula of this compound is C18H33ClN2O5S, and its molecular weight is 424.98 g/mol. []
Q4: How stable are intravenous admixtures containing this compound?
A6: Intravenous admixtures of this compound phosphate with aztreonam at specific concentrations have demonstrated stability for at least 48 hours at 22-23°C and for at least seven days at 4°C. []
Q5: How do structural modifications of this compound affect its activity?
A7: Research shows that the presence of the 7(S)-chloro-7-deoxy function in this compound is crucial for its potent antibacterial activity. [] Alterations to this specific structural feature can significantly impact this compound's ability to bind to the bacterial ribosome and inhibit protein synthesis. []
Q6: What are some strategies to enhance this compound's delivery to target tissues?
A8: One approach involves encapsulating this compound phosphate into transfersomal nanoparticles. [] These nanoparticles have been shown to improve the drug's transdermal delivery and penetration into deeper skin layers, potentially enhancing its efficacy for treating skin infections. []
Q7: Can bile acids improve this compound's permeation through the skin?
A9: Yes, incorporating bile acids like cholic acid into this compound hydrogel formulations has been shown to enhance the drug's release rate and permeation through cellulose membranes in vitro. [] This finding suggests the potential for bile acids to improve this compound's penetration through the skin barrier. []
Q8: How is this compound absorbed and distributed in the body?
A10: this compound is rapidly absorbed after oral administration, reaching peak plasma concentrations in about 0.5 hours. [] It demonstrates good tissue penetration and is known to accumulate in phagocytes, which can be beneficial for treating intracellular infections. [, ]
Q9: Are there differences in bioavailability between this compound phosphate ester tablets and this compound hydrochloride capsules?
A11: Studies have shown bioequivalence between orally disintegrating this compound phosphate ester tablets and this compound hydrochloride capsules. [] This suggests that both formulations provide comparable amounts of this compound in the bloodstream. []
Q10: What are some animal models used to study this compound's efficacy?
A12: A murine model has been utilized to investigate this compound's efficacy in treating community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) pneumonia. [] Results indicated that this compound effectively reduced bacterial load, improved survival rates, and mitigated lung damage in infected mice. []
Q11: Has this compound been compared to other antibiotics in clinical trials?
A13: Yes, several clinical trials have compared this compound to other antibiotics for treating various infections. For instance, a study on bacterial vaginosis found comparable cure rates between oral metronidazole, metronidazole vaginal gel, and this compound vaginal cream. [] Another trial showed similar efficacy between this compound, amoxicillin, and erythromycin for treating Chlamydia trachomatis in pregnant women. []
Q12: What is the significance of inducible this compound resistance in Staphylococcal infections?
A14: Inducible this compound resistance (ICR) is a concern in Staphylococcal infections because routine susceptibility tests may misinterpret resistant strains as susceptible. [, , , , , , , ] This misinterpretation can lead to therapeutic failure if this compound is chosen for treatment. [, , , , , , , ] Performing a D-test is crucial to accurately identify ICR and guide appropriate antibiotic selection. [, , , , , , , ]
Q13: What is the most common mechanism behind this compound resistance in Staphylococci?
A15: The most prevalent mechanism is through the erm gene, which encodes for ribosomal methylases. [, , , , , , , ] These methylases modify the bacterial ribosome, preventing this compound binding and rendering the bacteria resistant. [, , , , , , , ]
Q14: How does prior this compound exposure affect the susceptibility of Clostridium difficile?
A16: Studies indicate that prior this compound use is significantly associated with Clostridium difficile-associated diarrhea (CDAD) caused by isolates resistant to this compound, erythromycin, and trovafloxacin. [] This association highlights the potential risk of selecting for resistant C. difficile strains following this compound exposure. []
Q15: What analytical methods are commonly used to quantify this compound?
A17: High-performance liquid chromatography (HPLC) coupled with UV detection is a widely used method for quantifying this compound in various matrices. [, , ] UPLC-MS (Ultra Performance Liquid Chromatography-Mass Spectrometry) is another powerful technique used to detect and quantify this compound and its related substances, even at low concentrations. []
Q16: How can researchers ensure the accuracy and reliability of their analytical methods for this compound?
A18: Rigorous analytical method validation is essential. This process involves assessing parameters such as accuracy, precision, specificity, linearity, range, limit of detection, limit of quantitation, robustness, and system suitability. [] Validated methods ensure reliable and accurate data for this compound analysis. []
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.