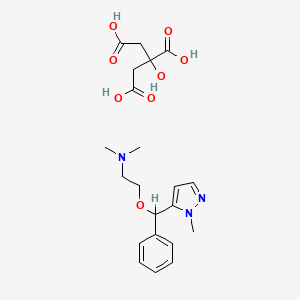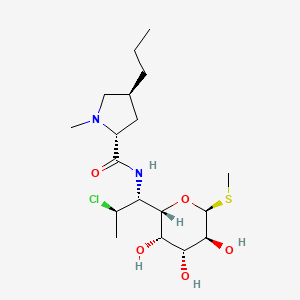
クリンダマイシン
概要
説明
クリンダマイシンは、さまざまな細菌感染症の治療に使用されるリンコサミド系抗生物質です。特に、嫌気性菌や特定のグラム陽性菌(ブドウ球菌や連鎖球菌など)に対して有効です。 クリンダマイシンは、骨髄炎、骨盤内炎症性疾患、咽頭炎、肺炎、皮膚感染症などの感染症の治療に一般的に使用されます 。 経口カプセル、外用クリーム、静脈注射液など、さまざまな形態で入手できます .
2. 製法
クリンダマイシンは、天然に存在する抗生物質であるリンコマイシンから合成されます。 合成には、リンコマイシンの7位のヒドロキシル基を塩素原子に置換する塩素化が伴います 。このプロセスには、いくつかの段階が含まれます。
シリコン保護基の付加: リンコマイシンは、最初にシリコン基を使用して保護されます。
選択的脱保護: 保護されたリンコマイシンは、選択的脱保護を受けます。
ミツノブ反応: 脱保護されたリンコマイシンは、ミツノブ反応を受けます。
加水分解反応: その後、生成物は加水分解されて7-エピメリンコマイシンが得られます。
クリンダマイシン塩酸塩の工業生産には、塩素化、加水分解、抽出、濃縮の段階が含まれ、遊離アルカリ形態が得られます。その後、塩形成と脱アルコール化を行い、クリンダマイシン塩酸塩が得られます .
3. 化学反応の分析
クリンダマイシンは、さまざまな化学反応を起こし、それらには以下が含まれます。
酸化: クリンダマイシンは、酸化されてスルホキシドとN-脱メチル化代謝物を形成することができます。
還元: 還元反応はそれほど一般的ではありませんが、特定の条件下で起こる可能性があります。
これらの反応に使用される一般的な試薬には、塩素化剤、酸化剤、還元剤が含まれます。 これらの反応から生成される主な生成物は、クリンダマイシン塩酸塩とその代謝物です .
4. 科学研究への応用
クリンダマイシンは、幅広い科学研究に利用されています。
化学: 抗生物質の合成と改変を研究するためのモデル化合物として使用されます。
生物学: クリンダマイシンは、細菌のタンパク質合成と耐性メカニズムの研究に使用されます。
作用機序
クリンダマイシンは、細菌の50Sリボソームサブユニットに結合することにより、タンパク質合成を阻害して作用します。 この作用は、翻訳中のペプチド鎖の伸長を阻害し、細菌の増殖を効果的に停止します 。 クリンダマイシンは細菌のリボソームを標的とし、トランスペプチダーゼ反応を阻害し、初期の鎖伸長を阻害します .
科学的研究の応用
Clindamycin has a wide range of scientific research applications:
Chemistry: It is used as a model compound in studying antibiotic synthesis and modification.
Biology: Clindamycin is used to study bacterial protein synthesis and resistance mechanisms.
Medicine: It is widely used to treat bacterial infections, including those caused by methicillin-resistant Staphylococcus aureus (MRSA) and anaerobic bacteria
生化学分析
Biochemical Properties
Clindamycin works primarily by binding to the 50S ribosomal subunit of bacteria . This agent disrupts protein synthesis by interfering with the transpeptidation reaction, which thereby inhibits early chain elongation . By disrupting bacterial protein synthesis, clindamycin causes changes in the cell wall surface, which decreases adherence of bacteria to host cells and increases intracellular killing of organisms .
Cellular Effects
Clindamycin achieves high intracellular levels in phagocytic cells . By disrupting bacterial protein synthesis, clindamycin causes changes in the cell wall surface, which decreases adherence of bacteria to host cells and increases intracellular killing of organisms .
Molecular Mechanism
Clindamycin inhibits bacterial protein synthesis by binding to 23S RNA of the 50S subunit of the bacterial ribosome . It impedes both the assembly of the ribosome and the translation process . The molecular mechanism through which this occurs is thought to be due to clindamycin’s three-dimensional structure, which closely resembles the 3’-ends of L-Pro-Met-tRNA and deacylated-tRNA during the peptide elongation cycle .
Temporal Effects in Laboratory Settings
Clindamycin exerts an extended postantibiotic effect against some strains of bacteria, which may be attributed to persistence of the drug at the ribosomal binding site .
Dosage Effects in Animal Models
In veterinary medicine, clindamycin is used at a dosage of 10-15 mg/kg, administered orally or intravenously every 12-24 hours . There are rarely reported cases of overdosage since clindamycin is well tolerated even at high dosages .
Metabolic Pathways
Clindamycin undergoes hepatic metabolism mediated primarily by CYP3A4 and, to a lesser extent, CYP3A5 . Two inactive metabolites have been identified - an oxidative metabolite, clindamycin sulfoxide, and an N-demethylated metabolite, N-desmethylclindamycin .
Transport and Distribution
Clindamycin is widely distributed in the body, including into bone, but does not distribute into cerebrospinal fluid . The volume of distribution has been variably estimated between 43-74 L .
Subcellular Localization
The primary targets of clindamycin, the 50S ribosomal subunits, are located in the cytoplasm of the bacterial cell . Therefore, the subcellular localization of clindamycin would be within the bacterial cytoplasm where it can exert its effects .
準備方法
Clindamycin is synthesized from lincomycin, a naturally occurring antibiotic. The synthesis involves the chlorination of lincomycin to replace the hydroxyl group at position 7 with a chlorine atom . The process includes several steps:
Silicon Protecting Group Application: Lincomycin is first protected using a silicon group.
Selective Deprotection: The protected lincomycin undergoes selective deprotection.
Mitsunobu Substitution Reaction: The deprotected lincomycin is subjected to a Mitsunobu substitution reaction.
Hydrolysis Reaction: The product is then hydrolyzed to obtain 7-epime lincomycin.
Chlorination Reaction: Finally, the 7-epime lincomycin is chlorinated to produce clindamycin.
Industrial production of clindamycin hydrochloride involves chlorination, hydrolysis, extraction, and concentration steps to obtain the free alkali form, followed by salt formation and dealcoholation to yield clindamycin hydrochloride .
化学反応の分析
Clindamycin undergoes various chemical reactions, including:
Oxidation: Clindamycin can be oxidized to form sulfoxide and N-demethylated metabolites.
Reduction: Reduction reactions are less common but can occur under specific conditions.
Substitution: The Mitsunobu substitution reaction is a key step in its synthesis.
Common reagents used in these reactions include chlorinating agents, oxidizing agents, and reducing agents. The major products formed from these reactions are clindamycin hydrochloride and its metabolites .
類似化合物との比較
クリンダマイシンは、多くの場合、以下のような他の抗生物質と比較されます。
リンコマイシン: クリンダマイシンは、リンコマイシンの塩素化誘導体であり、より優れた特性を持っています。
アモキシシリン: 同様の感染症に用いられるペニシリン系抗生物質ですが、作用機序が異なります。
ドキシサイクリン: より幅広い活性を持つテトラサイクリン系抗生物質です
クリンダマイシンは、嫌気性菌に対する高い有効性と骨や膿瘍への浸透能力が高く、骨髄炎やその他の深在性感染症の治療に特に有効である点が特徴です .
特性
IUPAC Name |
(2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KDLRVYVGXIQJDK-AWPVFWJPSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCC1CC(N(C1)C)C(=O)NC(C2C(C(C(C(O2)SC)O)O)O)C(C)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCC[C@@H]1C[C@H](N(C1)C)C(=O)N[C@@H]([C@@H]2[C@@H]([C@@H]([C@H]([C@H](O2)SC)O)O)O)[C@H](C)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H33ClN2O5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
21462-39-5 (mono-hydrochloride), 58207-19-5 (mono-HCl, mono-hydrate) | |
| Record name | Clindamycin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0018323449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID2022836 | |
| Record name | Clindamycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
425.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Clindamycin may be bacteriostatic or bactericidal in action, depending on the concentration of the drug attained at the site of infection and the susceptibility of the infecting organism. Clindamycin palmitate hydrochloride and clindamycin phosphate are inactive until hydrolyzed to free clindamycin. This hydrolysis occurs rapidly in vivo. Clindamycin appears to inhibit protein synthesis in susceptible organisms by binding to 50S ribosomal subunits; the primary effect is inhibition of peptide bond formation. The site of action appears to be the same as that of erythromycin, chloramphenicol, and lincomycin., Clindamycin binds exclusively to the 50S subunit of bacterial ribosomes and suppresses protein synthesis., ... Clindamycin is not a substrate for macrolide efflux pumps, and strains that are resistant to macrolides by this mechanism are susceptible to clindamycin. | |
| Record name | CLINDAMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3037 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow, amorphous solid | |
CAS No. |
18323-44-9 | |
| Record name | Clindamycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=18323-44-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clindamycin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0018323449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clindamycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clindamycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.038.357 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLINDAMYCIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3U02EL437C | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLINDAMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3037 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Clindamycin exert its antibacterial effect?
A1: Clindamycin inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit, specifically at the center of the peptidyl transferase center. [, , ] This binding prevents peptide bond formation and disrupts the translocation process, ultimately halting protein synthesis and leading to bacterial growth inhibition or death. [, ]
Q2: Does Clindamycin possess any anti-inflammatory properties?
A3: Yes, in addition to its antibacterial action, Clindamycin also exhibits anti-inflammatory effects. [] It has been shown to reduce inflammation associated with acne by influencing inflammatory pathways and potentially inhibiting neutrophil chemotaxis. []
Q3: What is the molecular formula and weight of Clindamycin?
A4: The molecular formula of Clindamycin is C18H33ClN2O5S, and its molecular weight is 424.98 g/mol. []
Q4: How stable are intravenous admixtures containing Clindamycin?
A6: Intravenous admixtures of Clindamycin phosphate with aztreonam at specific concentrations have demonstrated stability for at least 48 hours at 22-23°C and for at least seven days at 4°C. []
Q5: How do structural modifications of Clindamycin affect its activity?
A7: Research shows that the presence of the 7(S)-chloro-7-deoxy function in Clindamycin is crucial for its potent antibacterial activity. [] Alterations to this specific structural feature can significantly impact Clindamycin's ability to bind to the bacterial ribosome and inhibit protein synthesis. []
Q6: What are some strategies to enhance Clindamycin's delivery to target tissues?
A8: One approach involves encapsulating Clindamycin phosphate into transfersomal nanoparticles. [] These nanoparticles have been shown to improve the drug's transdermal delivery and penetration into deeper skin layers, potentially enhancing its efficacy for treating skin infections. []
Q7: Can bile acids improve Clindamycin's permeation through the skin?
A9: Yes, incorporating bile acids like cholic acid into Clindamycin hydrogel formulations has been shown to enhance the drug's release rate and permeation through cellulose membranes in vitro. [] This finding suggests the potential for bile acids to improve Clindamycin's penetration through the skin barrier. []
Q8: How is Clindamycin absorbed and distributed in the body?
A10: Clindamycin is rapidly absorbed after oral administration, reaching peak plasma concentrations in about 0.5 hours. [] It demonstrates good tissue penetration and is known to accumulate in phagocytes, which can be beneficial for treating intracellular infections. [, ]
Q9: Are there differences in bioavailability between Clindamycin phosphate ester tablets and Clindamycin hydrochloride capsules?
A11: Studies have shown bioequivalence between orally disintegrating Clindamycin phosphate ester tablets and Clindamycin hydrochloride capsules. [] This suggests that both formulations provide comparable amounts of Clindamycin in the bloodstream. []
Q10: What are some animal models used to study Clindamycin's efficacy?
A12: A murine model has been utilized to investigate Clindamycin's efficacy in treating community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) pneumonia. [] Results indicated that Clindamycin effectively reduced bacterial load, improved survival rates, and mitigated lung damage in infected mice. []
Q11: Has Clindamycin been compared to other antibiotics in clinical trials?
A13: Yes, several clinical trials have compared Clindamycin to other antibiotics for treating various infections. For instance, a study on bacterial vaginosis found comparable cure rates between oral metronidazole, metronidazole vaginal gel, and Clindamycin vaginal cream. [] Another trial showed similar efficacy between Clindamycin, amoxicillin, and erythromycin for treating Chlamydia trachomatis in pregnant women. []
Q12: What is the significance of inducible clindamycin resistance in Staphylococcal infections?
A14: Inducible clindamycin resistance (ICR) is a concern in Staphylococcal infections because routine susceptibility tests may misinterpret resistant strains as susceptible. [, , , , , , , ] This misinterpretation can lead to therapeutic failure if Clindamycin is chosen for treatment. [, , , , , , , ] Performing a D-test is crucial to accurately identify ICR and guide appropriate antibiotic selection. [, , , , , , , ]
Q13: What is the most common mechanism behind clindamycin resistance in Staphylococci?
A15: The most prevalent mechanism is through the erm gene, which encodes for ribosomal methylases. [, , , , , , , ] These methylases modify the bacterial ribosome, preventing Clindamycin binding and rendering the bacteria resistant. [, , , , , , , ]
Q14: How does prior clindamycin exposure affect the susceptibility of Clostridium difficile?
A16: Studies indicate that prior clindamycin use is significantly associated with Clostridium difficile-associated diarrhea (CDAD) caused by isolates resistant to clindamycin, erythromycin, and trovafloxacin. [] This association highlights the potential risk of selecting for resistant C. difficile strains following clindamycin exposure. []
Q15: What analytical methods are commonly used to quantify Clindamycin?
A17: High-performance liquid chromatography (HPLC) coupled with UV detection is a widely used method for quantifying Clindamycin in various matrices. [, , ] UPLC-MS (Ultra Performance Liquid Chromatography-Mass Spectrometry) is another powerful technique used to detect and quantify Clindamycin and its related substances, even at low concentrations. []
Q16: How can researchers ensure the accuracy and reliability of their analytical methods for Clindamycin?
A18: Rigorous analytical method validation is essential. This process involves assessing parameters such as accuracy, precision, specificity, linearity, range, limit of detection, limit of quantitation, robustness, and system suitability. [] Validated methods ensure reliable and accurate data for Clindamycin analysis. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![(3-hydroxy-23,24-dimethoxy-7,7-dimethyl-5,8,18,27,29-pentaoxo-9,20-dioxa-6-azaheptacyclo[15.12.0.02,14.04,12.06,10.019,28.021,26]nonacosa-1(17),2,4(12),13,15,19(28),21,23,25-nonaen-10-yl)methyl 3-methylbutanoate](/img/structure/B1669104.png)