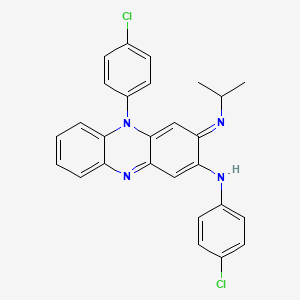
Clofazimin
Übersicht
Beschreibung
Clofazimine is a highly lipophilic antimicrobial riminophenazine dye used primarily in the treatment of leprosy. It was first described in 1957 and is known for its bright-red color, which can cause long-lasting discoloration of the skin and bodily fluids . Clofazimine is used in combination with other agents, such as dapsone, to treat lepromatous leprosy, including dapsone-resistant cases .
Wirkmechanismus
Target of Action
Clofazimine primarily targets Mycobacterium leprae , the bacterium responsible for leprosy . It is believed to act on the bacterial outer membrane, the bacterial respiratory chain, and ion transporters . It also has anti-inflammatory properties due to its suppression of T-lymphocyte activity .
Mode of Action
Clofazimine exerts a slow bactericidal effect on Mycobacterium leprae due to its action on the bacterial outer membrane . It is also suggested that it interferes with DNA . The anti-inflammatory activity of clofazimine is the result of its inhibition of T-lymphocyte activation and proliferation . Several mechanisms have been proposed, including direct antagonism of T-cell Kv 1.3 potassium channels and indirect action by promoting the release of E-series prostaglandins and reactive oxygen species .
Biochemical Pathways
It is known to interfere with cellular respiration and ion transport in mycobacterium leprae . An in vitro study identified eight metabolites of clofazimine and the enzymatic pathways involved in their formation, including the important cytochrome P450 isoenzymes CYP3A4/A5 and CYP1A2 .
Pharmacokinetics
Clofazimine is a highly lipophilic antimicrobial, which allows it to accumulate in skin and nerves . It has a relatively long duration of action owing to its long residence time in the body . The pharmacokinetics of clofazimine were well characterized by a three-compartment model, with a clearance of 11.5 L/h and peripheral volume of 10,500 L for a typical participant . Lower plasma exposures were observed in women during the first few months of treatment, explained by higher body fat fraction .
Result of Action
The bactericidal effect of clofazimine results in the elimination of Mycobacterium leprae from the body . Its anti-inflammatory properties help control harmful erythema nodosum leprosum and reversal immunity reactions, which may complicate antimicrobial chemotherapy .
Action Environment
The action of clofazimine can be influenced by environmental factors. For instance, its lipophilic nature allows it to accumulate in fatty tissues, which can affect its distribution and efficacy . Furthermore, its pharmacokinetics and resulting efficacy can be influenced by the patient’s body fat content .
Wissenschaftliche Forschungsanwendungen
Clofazimin hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung, darunter:
Chemie: Wird als Modellverbindung für die Untersuchung lipophiler antimikrobieller Wirkstoffe verwendet.
Biologie: Untersucht wurden seine Auswirkungen auf bakterielle DNA und Membranphospholipide.
Medizin: Hauptsächlich zur Behandlung von Lepra und multiresistenter Tuberkulose eingesetzt.
Industrie: Wird bei der Entwicklung neuartiger Arzneimittelabgabesysteme wie Nanosuspensionen eingesetzt.
5. Wirkmechanismus
This compound wirkt, indem es an die Guaninbasen der bakteriellen DNA bindet, wodurch die Template-Funktion der DNA blockiert und die bakterielle Proliferation gehemmt wird . Es erhöht auch die Aktivität der bakteriellen Phospholipase A2, was zur Freisetzung und Anhäufung von Lysophospholipiden führt, die toxisch sind und die bakterielle Proliferation hemmen . Darüber hinaus zeigt this compound entzündungshemmende Eigenschaften, indem es die Aktivität von T-Lymphozyten unterdrückt .
Ähnliche Verbindungen:
Dapson: Ein weiteres antimikrobielles Mittel, das zur Behandlung von Lepra eingesetzt wird.
Rifampicin: Wird in Kombination mit this compound zur Behandlung von Lepra und Tuberkulose eingesetzt.
Einzigartigkeit von this compound: this compound ist einzigartig aufgrund seiner doppelten antimikrobiellen und entzündungshemmenden Eigenschaften sowie seiner Fähigkeit, sich in Haut und Nerven anzureichern, was es besonders wirksam bei der Behandlung von Lepra macht .
Biochemische Analyse
Biochemical Properties
Clofazimine exerts a slow bactericidal effect on Mycobacterium leprae due to its action on the bacterial outer membrane . There is also evidence that it affects the bacterial respiratory chain and ion transporters . Clofazimine is at least partially metabolized in the liver . An in vitro study using human liver microsomes identified eight metabolites of clofazimine and the enzymatic pathways involved in their formation, including the important cytochrome P450 isoenzymes CYP3A4/A5 and CYP1A2 .
Cellular Effects
Clofazimine has been found to modulate the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages . It also exerts anti-inflammatory properties due to the suppression of T-lymphocyte activity . In HepaRG cells, clofazimine was a weak inducer of CYP3A4 at low concentrations, but inhibited CYP3A4 at therapeutic concentrations .
Molecular Mechanism
Clofazimine works by binding to the guanine bases of bacterial DNA, thereby blocking the template function of the DNA and inhibiting bacterial proliferation . It also increases the activity of bacterial phospholipase A2, leading to the release and accumulation of lysophospholipids, which are toxic and inhibit bacterial proliferation .
Temporal Effects in Laboratory Settings
In a study of patients with severe Mycobacterium avium complex pulmonary disease (MAC-PD), clofazimine demonstrated a relatively favorable efficacy, regardless of the maintenance dose . This effect was more pronounced when administered for a duration exceeding 6 months . In mice receiving clofazimine, the lungs’ bacterial load continued to grow during the first seven days of treatment .
Dosage Effects in Animal Models
In an orthotopic melanoma mouse model, clofazimine reduced tumor size by 90% . The specific effects of different dosages of clofazimine in animal models have not been extensively studied.
Metabolic Pathways
Clofazimine is involved in several metabolic pathways. It has been found to modulate the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages . It also affects the bacterial respiratory chain and ion transporters .
Transport and Distribution
Clofazimine is a potential substrate of uptake and efflux transporters that might be involved in its disposition . The intracellular concentrations of clofazimine were significantly increased in the presence of selective inhibitors of P-gp and BCRP .
Subcellular Localization
Clofazimine has been found to accumulate in macrophages in an intracellular liquid crystal-like structure This suggests that clofazimine may be localized in specific subcellular compartments within these cells
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Clofazimine is synthesized through a multi-step process involving the condensation of 3-chloro-4-nitroaniline with 4-chlorobenzaldehyde to form a Schiff base. This intermediate is then cyclized to form the phenazine core structure. The final step involves the reduction of the nitro group to an amine .
Industrial Production Methods: Industrial production of clofazimine involves high-pressure homogenization to produce nanosuspensions suitable for intravenous use. This method ensures that the particle size is appropriate for passive targeting to the reticuloendothelial system .
Analyse Chemischer Reaktionen
Reaktionstypen: Clofazimin durchläuft verschiedene chemische Reaktionen, darunter Oxidation, Reduktion und Substitution. Es ist bekannt, dass es mit Membranphospholipiden interagiert, was zur Bildung antimikrobieller Lysophospholipide führt .
Häufige Reagenzien und Bedingungen: Häufige Reagenzien, die bei der Synthese und Reaktion von this compound verwendet werden, sind 3-Chlor-4-nitroanilin, 4-Chlorbenzaldehyd und Reduktionsmittel für den letzten Schritt .
Hauptprodukte: Das Hauptprodukt, das aus der Synthese von this compound gebildet wird, ist die Riminophenazin-Kernstruktur, die für seine antimikrobielle Aktivität unerlässlich ist .
Vergleich Mit ähnlichen Verbindungen
Dapsone: Another antimicrobial agent used in the treatment of leprosy.
Rifampin: Used in combination with clofazimine for the treatment of leprosy and tuberculosis.
Uniqueness of Clofazimine: Clofazimine is unique due to its dual antimicrobial and anti-inflammatory properties, as well as its ability to accumulate in skin and nerves, making it particularly effective in treating leprosy .
Eigenschaften
IUPAC Name |
N,5-bis(4-chlorophenyl)-3-propan-2-yliminophenazin-2-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H22Cl2N4/c1-17(2)30-24-16-27-25(15-23(24)31-20-11-7-18(28)8-12-20)32-22-5-3-4-6-26(22)33(27)21-13-9-19(29)10-14-21/h3-17,31H,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WDQPAMHFFCXSNU-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)N=C1C=C2C(=NC3=CC=CC=C3N2C4=CC=C(C=C4)Cl)C=C1NC5=CC=C(C=C5)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H22Cl2N4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7022839 | |
| Record name | Clofazimine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022839 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
473.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
0.3 [ug/mL] (The mean of the results at pH 7.4), 1.51e-03 g/L | |
| Record name | SID49681815 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Although the precise mechanism(s) of action of clofazimine have not been elucidated, its antimicrobial activity appears to be membrane-directed. It was previously thought that, due to its lipophilicity, clofazimine participated in the generation of intracellular reactive oxygen species (ROS) via redox cycling, specifically H2O2 and superoxide, which then exerted an antimicrobial effect. A more recent and compelling theory involves clofazimine interacting with bacterial membrane phospholipids to generate antimicrobial lysophospholipids - bactericidal efficacy may, then, arise from the combined membrane-destabilizing effects of both clofazimine and lysophospholipids, which interfere with K+ uptake and, ultimately, ATP production. The anti-inflammatory activity of clofazimine is the result of its inhibition of T-lymphocyte activation and proliferation. Several mechanisms have been proposed, including direct antagonism of T-cell Kv 1.3 potassium channels and indirect action by promoting the release of E-series prostaglandins and reactive oxygen species from bystander neutrophils and monocytes. | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
2030-63-9 | |
| Record name | Clofazimine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=2030-63-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clofazimine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002030639 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clofazimine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759283 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | clofazimine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=141046 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clofazimine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022839 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clofazimine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.016.347 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOFAZIMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/D959AE5USF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
210-212 °C, 210 - 212 °C | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![2-Propylthiazolo[4,5-c]quinolin-4-amine](/img/structure/B1669137.png)
