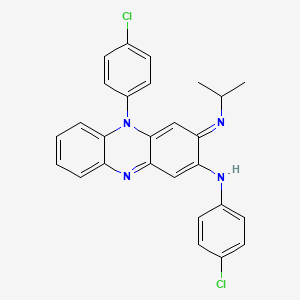
クロファジミン
概要
説明
クロファジミンは、主にらい菌の治療に使用される、高度に脂溶性の抗菌性リミノフェナジン染料です。 クロファジミンは、1957年に初めて記載され、その鮮やかな赤色で知られています。この色は、皮膚や体液に長期間色素沈着を引き起こす可能性があります 。 クロファジミンは、ダプソンなどの他の薬剤と組み合わせて、ダプソン耐性を含むらい菌の治療に使用されます .
作用機序
科学的研究の応用
クロファジミンは、以下のものを含む、さまざまな科学研究における応用があります。
生化学分析
Biochemical Properties
Clofazimine exerts a slow bactericidal effect on Mycobacterium leprae due to its action on the bacterial outer membrane . There is also evidence that it affects the bacterial respiratory chain and ion transporters . Clofazimine is at least partially metabolized in the liver . An in vitro study using human liver microsomes identified eight metabolites of clofazimine and the enzymatic pathways involved in their formation, including the important cytochrome P450 isoenzymes CYP3A4/A5 and CYP1A2 .
Cellular Effects
Clofazimine has been found to modulate the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages . It also exerts anti-inflammatory properties due to the suppression of T-lymphocyte activity . In HepaRG cells, clofazimine was a weak inducer of CYP3A4 at low concentrations, but inhibited CYP3A4 at therapeutic concentrations .
Molecular Mechanism
Clofazimine works by binding to the guanine bases of bacterial DNA, thereby blocking the template function of the DNA and inhibiting bacterial proliferation . It also increases the activity of bacterial phospholipase A2, leading to the release and accumulation of lysophospholipids, which are toxic and inhibit bacterial proliferation .
Temporal Effects in Laboratory Settings
In a study of patients with severe Mycobacterium avium complex pulmonary disease (MAC-PD), clofazimine demonstrated a relatively favorable efficacy, regardless of the maintenance dose . This effect was more pronounced when administered for a duration exceeding 6 months . In mice receiving clofazimine, the lungs’ bacterial load continued to grow during the first seven days of treatment .
Dosage Effects in Animal Models
In an orthotopic melanoma mouse model, clofazimine reduced tumor size by 90% . The specific effects of different dosages of clofazimine in animal models have not been extensively studied.
Metabolic Pathways
Clofazimine is involved in several metabolic pathways. It has been found to modulate the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages . It also affects the bacterial respiratory chain and ion transporters .
Transport and Distribution
Clofazimine is a potential substrate of uptake and efflux transporters that might be involved in its disposition . The intracellular concentrations of clofazimine were significantly increased in the presence of selective inhibitors of P-gp and BCRP .
Subcellular Localization
Clofazimine has been found to accumulate in macrophages in an intracellular liquid crystal-like structure This suggests that clofazimine may be localized in specific subcellular compartments within these cells
準備方法
合成ルートと反応条件: クロファジミンは、3-クロロ-4-ニトロアニリンと4-クロロベンズアルデヒドを縮合させてシッフ塩基を形成する、複数段階のプロセスを経て合成されます。この中間体は環化してフェナジンコア構造を形成します。 最後の段階では、ニトロ基をアミンに還元します .
工業的生産方法: クロファジミンの工業的生産には、静脈内投与に適したナノ懸濁液を生成するために、高圧均質化が用いられます。 この方法は、粒子サイズがレチクログロビュール系への受動的ターゲティングに適していることを保証します .
化学反応の分析
反応の種類: クロファジミンは、酸化、還元、置換など、さまざまな化学反応を受けます。 クロファジミンは、膜リン脂質と相互作用することが知られており、抗菌性リゾリン脂質の生成につながります .
一般的な試薬と条件: クロファジミンの合成と反応に使用される一般的な試薬には、3-クロロ-4-ニトロアニリン、4-クロロベンズアルデヒド、最後の段階での還元剤が含まれます .
主要な生成物: クロファジミンの合成から生成される主な生成物は、リミノフェナジンコア構造であり、その抗菌活性に不可欠です .
類似化合物との比較
Dapsone: Another antimicrobial agent used in the treatment of leprosy.
Rifampin: Used in combination with clofazimine for the treatment of leprosy and tuberculosis.
Uniqueness of Clofazimine: Clofazimine is unique due to its dual antimicrobial and anti-inflammatory properties, as well as its ability to accumulate in skin and nerves, making it particularly effective in treating leprosy .
特性
IUPAC Name |
N,5-bis(4-chlorophenyl)-3-propan-2-yliminophenazin-2-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H22Cl2N4/c1-17(2)30-24-16-27-25(15-23(24)31-20-11-7-18(28)8-12-20)32-22-5-3-4-6-26(22)33(27)21-13-9-19(29)10-14-21/h3-17,31H,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WDQPAMHFFCXSNU-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)N=C1C=C2C(=NC3=CC=CC=C3N2C4=CC=C(C=C4)Cl)C=C1NC5=CC=C(C=C5)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H22Cl2N4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7022839 | |
| Record name | Clofazimine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022839 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
473.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
0.3 [ug/mL] (The mean of the results at pH 7.4), 1.51e-03 g/L | |
| Record name | SID49681815 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Although the precise mechanism(s) of action of clofazimine have not been elucidated, its antimicrobial activity appears to be membrane-directed. It was previously thought that, due to its lipophilicity, clofazimine participated in the generation of intracellular reactive oxygen species (ROS) via redox cycling, specifically H2O2 and superoxide, which then exerted an antimicrobial effect. A more recent and compelling theory involves clofazimine interacting with bacterial membrane phospholipids to generate antimicrobial lysophospholipids - bactericidal efficacy may, then, arise from the combined membrane-destabilizing effects of both clofazimine and lysophospholipids, which interfere with K+ uptake and, ultimately, ATP production. The anti-inflammatory activity of clofazimine is the result of its inhibition of T-lymphocyte activation and proliferation. Several mechanisms have been proposed, including direct antagonism of T-cell Kv 1.3 potassium channels and indirect action by promoting the release of E-series prostaglandins and reactive oxygen species from bystander neutrophils and monocytes. | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
2030-63-9 | |
| Record name | Clofazimine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=2030-63-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clofazimine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002030639 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clofazimine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759283 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | clofazimine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=141046 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clofazimine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022839 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clofazimine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.016.347 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOFAZIMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/D959AE5USF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
210-212 °C, 210 - 212 °C | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![N-(Benzylsulfonyl)seryl-N~1~-{4-[(Z)-amino(imino)methyl]benzyl}serinamide](/img/structure/B1669119.png)
![5-iodo-2-(3-nitrophenyl)-1H-phenanthro[9,10-d]imidazole](/img/structure/B1669124.png)