Delamanid
Übersicht
Beschreibung
Delamanid is a nitro-dihydro-imidazooxazole derivative used primarily in the treatment of multidrug-resistant tuberculosis (MDR-TB). It is known for its potent in vitro and in vivo antitubercular activity against both drug-susceptible and drug-resistant strains of Mycobacterium tuberculosis . This compound is marketed under the trade name Deltyba and is approved for use in several countries, including Japan and those in the European Union .
Wissenschaftliche Forschungsanwendungen
Delamanid hat eine breite Palette an Anwendungen in der wissenschaftlichen Forschung, darunter:
Chemie: Wird als Modellverbindung zur Untersuchung von Nitroimidazol-Derivaten verwendet.
Biologie: Untersucht auf seine Auswirkungen auf Mycobacterium tuberculosis und andere Bakterienstämme.
Medizin: Wird hauptsächlich zur Behandlung von multiresistenten Tuberkulose eingesetzt. .
Industrie: Einsatz bei der Entwicklung neuer Antituberkulose-Medikamente und -Formulierungen.
5. Wirkmechanismus
This compound übt seine Wirkung aus, indem es die Synthese von Methoxy- und Keto-Mycolsäuren hemmt, die essentielle Bestandteile der Mycobakterien-Zellwand sind. Es ist ein Prodrug, das zur Aktivierung durch das mycobakterielle F420-Coenzymsystem, einschließlich der Deazaflavin-abhängigen Nitroreduktase, benötigt wird. Diese Aktivierung führt zur Bildung von reaktiven Stickstoffspezies, die die Synthese von Mycolsäuren stören und letztendlich die Bakterien abtöten .
Ähnliche Verbindungen:
Pretomanid: Ein weiteres Nitroimidazol-Derivat, das zur Behandlung von Tuberkulose eingesetzt wird.
Isoniazid: Ein Anti-Tuberkulose-Medikament der ersten Wahl, das die Mycolsäure-Synthese hemmt.
Rifampicin: Ein Antibiotikum, das die bakterielle RNA-Synthese hemmt.
Vergleich:
This compound vs. Pretomanid: This compound zeigt eine höhere in-vitro-Potenz gegen multiresistente und extensiv resistente Tuberkulose-Stämme im Vergleich zu Pretomanid.
This compound vs. Isoniazid: Obwohl beide die Mycolsäure-Synthese hemmen, hat this compound einen anderen Mechanismus, der das F420-Coenzymsystem beinhaltet.
This compound vs. Rifampicin: This compound zielt auf die Mycolsäure-Synthese ab, während Rifampicin die RNA-Synthese hemmt, wodurch sie sich in der Kombinationstherapie ergänzen
Der einzigartige Wirkmechanismus von this compound und seine Wirksamkeit gegen medikamentenresistente Stämme machen es zu einer wertvollen Ergänzung des Arsenals von Antituberkulose-Medikamenten.
Biochemische Analyse
Biochemical Properties
Delamanid inhibits the synthesis of methoxy-mycolic and keto-mycolic acid, which are essential components of the mycobacterial cell wall . This inhibition is achieved through the F420 coenzyme mycobacteria system, which generates nitrous oxide . The interaction between this compound and these biomolecules disrupts the integrity of the bacterial cell wall, thereby exerting its antimycobacterial properties .
Cellular Effects
This compound’s impact on cellular processes is primarily observed in its effects on Mycobacterium tuberculosis. By inhibiting the synthesis of key cell wall components, this compound disrupts the normal function of the bacteria, leading to their eventual death . This includes impacts on cell signaling pathways, gene expression, and cellular metabolism associated with the synthesis and maintenance of the cell wall .
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of methoxy- and keto-mycolic acid synthesis through the F420 coenzyme mycobacteria system . This system generates nitrous oxide, which contributes to the antimycobacterial properties of this compound . The active free radical produced by the mycobacterial F420-dependent nitroreductase coenzyme system is also a part of this compound’s mechanism of action .
Temporal Effects in Laboratory Settings
Its stability and long-term effects on cellular function have been observed in in vitro studies
Dosage Effects in Animal Models
Preliminary studies suggest that this compound has a dose-dependent effect on the treatment of MDR-TB
Metabolic Pathways
This compound is involved in the metabolic pathways related to the synthesis of mycolic acids . It interacts with the F420 coenzyme mycobacteria system, which plays a crucial role in these metabolic pathways
Transport and Distribution
It is known that this compound is primarily metabolized by albumin into the metabolite DM-6705
Subcellular Localization
Given its role in inhibiting the synthesis of cell wall components, it is likely that this compound localizes to areas of the cell where these synthesis processes occur
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Delamanid is synthesized through a multi-step process involving the formation of the imidazooxazole core followed by the introduction of various functional groups. The key steps include:
- Formation of the imidazooxazole ring.
- Introduction of the nitro group.
- Attachment of the trifluoromethoxyphenoxy and piperidinylphenoxy groups.
Industrial Production Methods: Industrial production of this compound involves large-scale synthesis using optimized reaction conditions to ensure high yield and purity. The process includes:
- Use of high-pressure homogenization for emulsification.
- Spray-drying to form a dried powder.
- Ensuring stability through nanoencapsulation to enhance aqueous dissolution kinetics .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Delamanid durchläuft verschiedene chemische Reaktionen, darunter:
Reduktion: Die Nitrogruppe wird zu einem Amin reduziert.
Oxidation: Der Imidazooxazol-Ring kann unter bestimmten Bedingungen oxidiert werden.
Substitution: Funktionelle Gruppen am Imidazooxazol-Ring können durch andere Gruppen substituiert werden.
Häufige Reagenzien und Bedingungen:
Reduktion: Katalytische Hydrierung unter Verwendung von Palladium auf Kohle.
Oxidation: Einsatz von Oxidationsmitteln wie Kaliumpermanganat.
Substitution: Nucleophile Substitutionsreaktionen unter Verwendung geeigneter Nucleophile.
Hauptprodukte:
- Die Reduktion der Nitrogruppe führt zur Bildung eines Amin-Derivats.
- Die Oxidation des Imidazooxazol-Rings kann verschiedene oxidierte Derivate hervorbringen.
- Substitutionsreaktionen ergeben eine Vielzahl von substituierten Imidazooxazol-Verbindungen .
Wirkmechanismus
Delamanid exerts its effects by inhibiting the synthesis of methoxy- and keto-mycolic acids, which are essential components of the mycobacterial cell wall. It is a prodrug that requires activation by the mycobacterial F420 coenzyme system, including the deazaflavin-dependent nitroreductase. This activation leads to the generation of reactive nitrogen species, which disrupt the synthesis of mycolic acids and ultimately kill the bacteria .
Vergleich Mit ähnlichen Verbindungen
Pretomanid: Another nitroimidazole derivative used in the treatment of tuberculosis.
Isoniazid: A first-line antitubercular drug that inhibits mycolic acid synthesis.
Rifampicin: An antibiotic that inhibits bacterial RNA synthesis.
Comparison:
Delamanid vs. Pretomanid: this compound exhibits greater in vitro potency against multidrug-resistant and extensively drug-resistant tuberculosis strains compared to pretomanid.
This compound vs. Isoniazid: While both inhibit mycolic acid synthesis, this compound has a distinct mechanism involving the F420 coenzyme system.
This compound vs. Rifampicin: this compound targets mycolic acid synthesis, whereas rifampicin inhibits RNA synthesis, making them complementary in combination therapy
This compound’s unique mechanism of action and its efficacy against drug-resistant strains make it a valuable addition to the arsenal of antitubercular drugs.
Eigenschaften
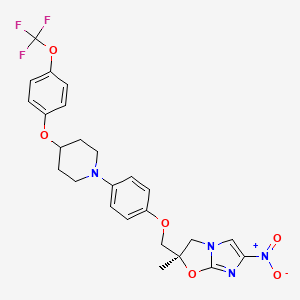
IUPAC Name |
(2R)-2-methyl-6-nitro-2-[[4-[4-[4-(trifluoromethoxy)phenoxy]piperidin-1-yl]phenoxy]methyl]-3H-imidazo[2,1-b][1,3]oxazole | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H25F3N4O6/c1-24(15-31-14-22(32(33)34)29-23(31)38-24)16-35-18-4-2-17(3-5-18)30-12-10-20(11-13-30)36-19-6-8-21(9-7-19)37-25(26,27)28/h2-9,14,20H,10-13,15-16H2,1H3/t24-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XDAOLTSRNUSPPH-XMMPIXPASA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1(CN2C=C(N=C2O1)[N+](=O)[O-])COC3=CC=C(C=C3)N4CCC(CC4)OC5=CC=C(C=C5)OC(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@@]1(CN2C=C(N=C2O1)[N+](=O)[O-])COC3=CC=C(C=C3)N4CCC(CC4)OC5=CC=C(C=C5)OC(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H25F3N4O6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60218326 | |
| Record name | Delamanid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60218326 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
534.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Delamanid is a prodrug that requires biotransformation via via the mycobacterial F420 coenzyme system, including the deazaflavin dependent nitroreductase (Rv3547), to mediate its antimycobacterial activity against both growing and nongrowing mycobacteria. Mutations in one of five coenzyme F420 genes, _fgd, Rv3547, fbiA, fbiB, and fbiC_ has been proposed as the mechanism of resistance to delamanid. Upon activation, the radical intermediate formed between delamanid and desnitro-imidazooxazole derivative is thought to mediate antimycobacterial actions via inhibition of methoxy-mycolic and keto-mycolic acid synthesis, leading to depletion of mycobacterial cell wall components and destruction of the mycobacteria. Nitroimidazooxazole derivative is thought to generate reactive nitrogen species, including nitrogen oxide (NO). However unlike isoniazid, delamanid does not alpha-mycolic acid. | |
| Record name | Delamanid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11637 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
681492-22-8 | |
| Record name | Delamanid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=681492-22-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Delamanid [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0681492228 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Delamanid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11637 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Delamanid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60218326 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | DELAMANID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/8OOT6M1PC7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.