Delamanid
Descripción general
Descripción
Delamanid es un derivado de nitro-dihidro-imidazooxazol utilizado principalmente en el tratamiento de la tuberculosis multirresistente (MDR-TB). Es conocido por su potente actividad antitubercular in vitro e in vivo contra cepas de Mycobacterium tuberculosis tanto sensibles como resistentes a los fármacos . This compound se comercializa bajo el nombre comercial Deltyba y está aprobado para su uso en varios países, incluidos Japón y los de la Unión Europea .
Análisis Bioquímico
Biochemical Properties
Delamanid inhibits the synthesis of methoxy-mycolic and keto-mycolic acid, which are essential components of the mycobacterial cell wall . This inhibition is achieved through the F420 coenzyme mycobacteria system, which generates nitrous oxide . The interaction between this compound and these biomolecules disrupts the integrity of the bacterial cell wall, thereby exerting its antimycobacterial properties .
Cellular Effects
This compound’s impact on cellular processes is primarily observed in its effects on Mycobacterium tuberculosis. By inhibiting the synthesis of key cell wall components, this compound disrupts the normal function of the bacteria, leading to their eventual death . This includes impacts on cell signaling pathways, gene expression, and cellular metabolism associated with the synthesis and maintenance of the cell wall .
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of methoxy- and keto-mycolic acid synthesis through the F420 coenzyme mycobacteria system . This system generates nitrous oxide, which contributes to the antimycobacterial properties of this compound . The active free radical produced by the mycobacterial F420-dependent nitroreductase coenzyme system is also a part of this compound’s mechanism of action .
Temporal Effects in Laboratory Settings
Its stability and long-term effects on cellular function have been observed in in vitro studies
Dosage Effects in Animal Models
Preliminary studies suggest that this compound has a dose-dependent effect on the treatment of MDR-TB
Metabolic Pathways
This compound is involved in the metabolic pathways related to the synthesis of mycolic acids . It interacts with the F420 coenzyme mycobacteria system, which plays a crucial role in these metabolic pathways
Transport and Distribution
It is known that this compound is primarily metabolized by albumin into the metabolite DM-6705
Subcellular Localization
Given its role in inhibiting the synthesis of cell wall components, it is likely that this compound localizes to areas of the cell where these synthesis processes occur
Métodos De Preparación
Rutas de síntesis y condiciones de reacción: Delamanid se sintetiza mediante un proceso de varios pasos que implica la formación del núcleo imidazooxazol seguido de la introducción de varios grupos funcionales. Los pasos clave incluyen:
- Formación del anillo imidazooxazol.
- Introducción del grupo nitro.
- Unión de los grupos trifluorometoxifenoxi y piperidinilfenoxi.
Métodos de producción industrial: La producción industrial de this compound implica la síntesis a gran escala utilizando condiciones de reacción optimizadas para garantizar un alto rendimiento y pureza. El proceso incluye:
- Uso de homogeneización a alta presión para la emulsificación.
- Secado por pulverización para formar un polvo seco.
- Asegurar la estabilidad mediante nanoencapsulación para mejorar la cinética de disolución acuosa .
Análisis De Reacciones Químicas
Tipos de reacciones: Delamanid experimenta varias reacciones químicas, que incluyen:
Reducción: El grupo nitro se reduce a una amina.
Oxidación: El anillo imidazooxazol puede sufrir oxidación en condiciones específicas.
Sustitución: Los grupos funcionales en el anillo imidazooxazol pueden ser sustituidos por otros grupos.
Reactivos y condiciones comunes:
Reducción: Hidrogenación catalítica utilizando paladio sobre carbono.
Oxidación: Uso de agentes oxidantes como el permanganato de potasio.
Sustitución: Reacciones de sustitución nucleofílica utilizando nucleófilos apropiados.
Productos principales:
- La reducción del grupo nitro conduce a la formación de un derivado de amina.
- La oxidación del anillo imidazooxazol puede producir varios derivados oxidados.
- Las reacciones de sustitución producen una variedad de compuestos imidazooxazol sustituidos .
Aplicaciones Científicas De Investigación
Delamanid tiene una amplia gama de aplicaciones de investigación científica, que incluyen:
Química: Utilizado como un compuesto modelo para estudiar derivados de nitroimidazol.
Biología: Investigado por sus efectos sobre Mycobacterium tuberculosis y otras cepas bacterianas.
Medicina: Principalmente utilizado en el tratamiento de la tuberculosis multirresistente. .
Industria: Empleado en el desarrollo de nuevos medicamentos y formulaciones antituberculosos.
Mecanismo De Acción
Delamanid ejerce sus efectos inhibiendo la síntesis de ácidos micólicos metoxilados y ceto-micólicos, que son componentes esenciales de la pared celular de las micobacterias. Es un profármaco que requiere activación por el sistema de coenzima F420 de las micobacterias, que incluye la nitroreductasa dependiente de deazaflavina. Esta activación conduce a la generación de especies reactivas de nitrógeno, que interrumpen la síntesis de ácidos micólicos y finalmente matan a las bacterias .
Compuestos similares:
Pretomanid: Otro derivado de nitroimidazol utilizado en el tratamiento de la tuberculosis.
Isoniazida: Un fármaco antitubercular de primera línea que inhibe la síntesis de ácidos micólicos.
Rifampicina: Un antibiótico que inhibe la síntesis de ARN bacteriano.
Comparación:
This compound vs. Pretomanid: this compound exhibe una mayor potencia in vitro contra cepas de tuberculosis multirresistentes y extremadamente resistentes a los medicamentos en comparación con pretomanid.
This compound vs. Isoniazida: Si bien ambos inhiben la síntesis de ácidos micólicos, this compound tiene un mecanismo distinto que involucra el sistema de coenzima F420.
This compound vs. Rifampicina: this compound se dirige a la síntesis de ácidos micólicos, mientras que la rifampicina inhibe la síntesis de ARN, lo que los hace complementarios en la terapia combinada
El mecanismo de acción único de this compound y su eficacia contra cepas resistentes a los medicamentos lo convierten en una adición valiosa al arsenal de medicamentos antituberculosos.
Comparación Con Compuestos Similares
Pretomanid: Another nitroimidazole derivative used in the treatment of tuberculosis.
Isoniazid: A first-line antitubercular drug that inhibits mycolic acid synthesis.
Rifampicin: An antibiotic that inhibits bacterial RNA synthesis.
Comparison:
Delamanid vs. Pretomanid: this compound exhibits greater in vitro potency against multidrug-resistant and extensively drug-resistant tuberculosis strains compared to pretomanid.
This compound vs. Isoniazid: While both inhibit mycolic acid synthesis, this compound has a distinct mechanism involving the F420 coenzyme system.
This compound vs. Rifampicin: this compound targets mycolic acid synthesis, whereas rifampicin inhibits RNA synthesis, making them complementary in combination therapy
This compound’s unique mechanism of action and its efficacy against drug-resistant strains make it a valuable addition to the arsenal of antitubercular drugs.
Propiedades
IUPAC Name |
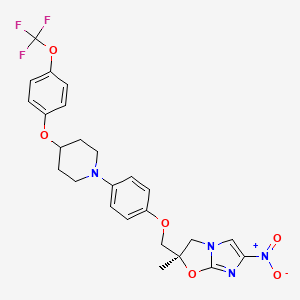
(2R)-2-methyl-6-nitro-2-[[4-[4-[4-(trifluoromethoxy)phenoxy]piperidin-1-yl]phenoxy]methyl]-3H-imidazo[2,1-b][1,3]oxazole | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H25F3N4O6/c1-24(15-31-14-22(32(33)34)29-23(31)38-24)16-35-18-4-2-17(3-5-18)30-12-10-20(11-13-30)36-19-6-8-21(9-7-19)37-25(26,27)28/h2-9,14,20H,10-13,15-16H2,1H3/t24-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XDAOLTSRNUSPPH-XMMPIXPASA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1(CN2C=C(N=C2O1)[N+](=O)[O-])COC3=CC=C(C=C3)N4CCC(CC4)OC5=CC=C(C=C5)OC(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@@]1(CN2C=C(N=C2O1)[N+](=O)[O-])COC3=CC=C(C=C3)N4CCC(CC4)OC5=CC=C(C=C5)OC(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H25F3N4O6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60218326 | |
| Record name | Delamanid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60218326 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
534.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Delamanid is a prodrug that requires biotransformation via via the mycobacterial F420 coenzyme system, including the deazaflavin dependent nitroreductase (Rv3547), to mediate its antimycobacterial activity against both growing and nongrowing mycobacteria. Mutations in one of five coenzyme F420 genes, _fgd, Rv3547, fbiA, fbiB, and fbiC_ has been proposed as the mechanism of resistance to delamanid. Upon activation, the radical intermediate formed between delamanid and desnitro-imidazooxazole derivative is thought to mediate antimycobacterial actions via inhibition of methoxy-mycolic and keto-mycolic acid synthesis, leading to depletion of mycobacterial cell wall components and destruction of the mycobacteria. Nitroimidazooxazole derivative is thought to generate reactive nitrogen species, including nitrogen oxide (NO). However unlike isoniazid, delamanid does not alpha-mycolic acid. | |
| Record name | Delamanid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11637 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
681492-22-8 | |
| Record name | Delamanid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=681492-22-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Delamanid [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0681492228 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Delamanid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11637 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Delamanid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60218326 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | DELAMANID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/8OOT6M1PC7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.