Ibuprofen
Übersicht
Beschreibung
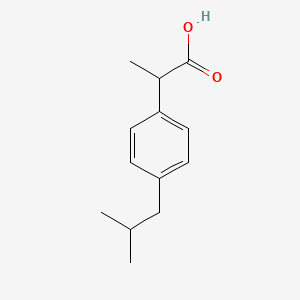
Ibuprofen, chemically known as 2-(4-isobutylphenyl)propanoic acid, is a widely used nonsteroidal anti-inflammatory drug (NSAID). It is primarily employed to relieve pain, reduce fever, and alleviate inflammation. Discovered in 1961 by Stewart Adams and John Nicholson, this compound was initially marketed under the brand name Brufen. Today, it is available under various brand names, including Advil, Motrin, and Nurofen .
Wirkmechanismus
The exact mechanism of action of ibuprofen is unknown. However, this compound is considered an NSAID and thus it is a non-selective inhibitor of cyclooxygenase, which is an enzyme involved in prostaglandin (mediators of pain and fever) and thromboxane (stimulators of blood clotting) synthesis via the arachidonic acid pathway. this compound is a non-selective COX inhibitor and hence, it inhibits the activity of both COX-1 and COX-2. The inhibition of COX-2 activity decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever, and swelling while the inhibition of COX-1 is thought to cause some of the side effects of this compound including GI ulceration.
This compound AT 25 MG/KG IV INCREASED THE PRIMARY AND TOTAL HEMOSTATIC PLUG FORMATION TIME IN RABBIT EAR CHAMBERS WITH LASER-INDUCED INJURY. THE SAME DOSE INCREASED THE NUMBER OF CUMULATIVE EMBOLI OVER A 10 MINUTE PERIOD AFTER A LASER INJURY TO ARTERIOLES. IN DOGS, DOSES OF 10, 25, AND 50 MG/KG DID NOT ENHANCE THE RELEASE OF (125)I-LABELED FIBRIN DEGRADATION PRODUCTS FROM THE THROMBI AFTER INCUBATION IN PLASMIN, BUT THE LARGEST DOSE SIGNIFICANTLY DECREASED THE THROMBUS WEIGHT 90 AND 180 MINUTES AFTER DRUG ADMINISTRATION. THUS, this compound HAD AN INHIBITORY EFFECT ON PLATELET FUNCTION IN VIVO AND IN LARGE DOSES DIMINISHED THE THROMBUS WEIGHT.
L-Arginine (L-arg) exhibits multiple biological properties and plays an important role in the regulation of different functions in pathological conditions. Many of these effects could be achieved on this amino acid serving as a substrate for the enzyme nitric oxide synthase (NOS). At the gastrointestinal level, recent reports revealed its protective activities involving a hyperemic response increasing the gastric blood flow. The aim of this study was to characterize the relationship between NOS activity/expression and prostaglandin changes (PGs) in rats gastric mucosa, with L-arg associated resistance to the nonsteroidal anti-inflammatory drug (NSAID) this compound (IBP). The protective effect of oral L-arg (100 mg/kg body wt), administerred together with IBP (100 mg/kg body wt, per os), was evident enough 90 min after drug administration, although a significant protection persisted for more than 6 hr. Pretreatment with N(G)-nitro-L-arginine (L-NNA) (40 mg/kg body wt, intraperitoneally), a competitive inhibitor of constitutive NOS, partly altered the protection afforded by the amino acid. In contrast, no changes could be observed after inducible NOS inhibition [aminoguanidine (AG) 50 mg/Kg body wt, intraperitoneally). L-arg, plus IBP, produced a significant increase of the cyclic GMP (cGMP) response in tissue samples from rat stomach, 90 min and 6 h after drug administration. iNOS activity and mRNA expression were higher in IBP-treated rats, and no differences were observed in inducible responses in the L-arg plus IBP group. No variations in the cNOS activity and expression were found among the different groups of animals assayed. The measurement of mucosal PGE2 content confirmed that biosynthesis of the eicosanoid is maintained by L-arg for over 90 min after IBP, while a total inhibition was observed 6 hr later. The mechanisms of the L-arg protective effect on the damaged induced by IBP could be explained by the different period after drug administration. The early phase is mediated by cyclooxygenase/prostaglandins pathway (COX/PGs) although NO liberated by cNOS and the guanylate cyclase/cGMP pathway could be also relevant. The later phase implicates inhibition of the iNOS/NO response.
We previously showed the non-steroidal anti-inflammatory drug (NSAID) this compound suppresses inflammation and amyloid in the APPsw (Tg2576) Tg2576 transgenic mouse. The mechanism for these effects and the impact on behavior are unknown. We now show this compound's effects were not mediated by alterations in amyloid precursor protein (APP) expression or oxidative damage (carbonyls). Six months this compound treatment in Tg+ females caused a decrease in open field behavior (p < 0.05), restoring values similar to Tg- mice. Reduced caspase activation per plaque provided further evidence for a neuroprotective action of this compound.The impact of a shorter 3 month duration this compound trial, beginning at a later age (from 14 to 17 months), was also investigated. Repeated measures ANOVA of Abeta levels (soluble and insoluble) demonstrated a significant this compound treatment effect (p < 0.05). Post-hoc analysis showed that this compound-dependent reductions of both soluble Abeta and Abeta42 were most marked in entorhinal cortex (p < 0.05). Although interleukin-1beta and insoluble Abeta were more effectively reduced with longer treatment, the magnitude of the effect on soluble Abeta was not dependent on treatment duration.
Trying to decrease the production of Amyloid beta (Abeta) has been envisaged as a promising approach to prevent neurodegeneration in Alzheimer's disease (AD). A chronic inflammatory reaction with activated microglia cells and astrocytes is a constant feature of AD. The participation of the immune system in the disease process is further documented in several retrospective clinical studies showing an inverse relationship between the prevalence of AD and nonsteroidal anti-inflammatory drug (NSAID) therapy. Previously, we demonstrated that the combination of the proinflammatory cytokines TNFalpha with IFNgamma induces the production of Abeta-42 and Abeta-40 in human neuronal cells. In the present study, the neuronal cell line Sk-n-sh was incubated for 12 h with the cyclooxygenase inhibitor this compound and subsequently stimulated with the cytokines TNFalpha and IFNgamma. This compound treatment decreased the secretion of total Abeta in the conditioned media of cytokine stimulated cells by 50% and prevented the accumulation of Abeta-42 and Abeta-40 in detergent soluble cell extracts. Viability of neuronal cells measured by detection of apoptosis was neither influenced by this compound nor by cytokine treatment. The reduction in the production of Abeta by this compound was presumably due to a decreased production of betaAPP, which in contrast to the control proteins M2 pyruvate kinase, beta-tubulin and the cytokine inducible ICAM-1 was detected at low concentration in this compound treated cells. The data demonstrate a possible mechanism how this compound may decrease the risk and delay the onset of AD.
Wissenschaftliche Forschungsanwendungen
Ibuprofen hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung in der Untersuchung von NSAR und deren Synthese.
Biologie: Untersucht wegen seiner Auswirkungen auf zelluläre Prozesse und Entzündungswege.
Medizin: Weitgehend erforscht wegen seiner therapeutischen Wirkungen bei der Behandlung von Schmerzen, Entzündungen und Fieber.
5. Wirkmechanismus
This compound übt seine Wirkung aus, indem es das Enzym Cyclooxygenase (COX) hemmt, das an der Synthese von Prostaglandinen beteiligt ist. Prostaglandine sind Lipidverbindungen, die Entzündungen, Schmerzen und Fieber vermitteln. Durch die Hemmung von COX reduziert this compound die Produktion von Prostaglandinen und lindert so diese Symptome. This compound ist ein nicht-selektiver COX-Hemmer, der sowohl COX-1- als auch COX-2-Enzyme beeinflusst .
Ähnliche Verbindungen:
- Aspirin (Acetylsalicylsäure)
- Naproxen (2-(6-Methoxynaphthalen-2-yl)propansäure)
- Diclofenac (2-(2,6-Dichloranilino)phenylessigsäure)
- Ketoprofen (2-(3-Benzoylphenyl)propansäure)
Vergleich:
- Aspirin: Wie this compound ist Aspirin ein nicht-selektiver COX-Hemmer, hat aber ein höheres Risiko für gastrointestinale Nebenwirkungen.
- Naproxen: Ähnlich wie this compound in seiner entzündungshemmenden Wirkung, hat aber eine längere Halbwertszeit, was eine weniger häufige Dosierung ermöglicht.
- Diclofenac: Wirksamer als this compound, aber mit höheren kardiovaskulären Risiken verbunden.
- Ketoprofen: In seiner Wirkung ähnlich wie this compound, kann aber mehr gastrointestinale Beschwerden verursachen .
This compound zeichnet sich durch sein ausgewogenes Wirksamkeits- und Sicherheitsprofil aus, was es zu einem der am häufigsten verwendeten NSAR weltweit macht.
Biochemische Analyse
Biochemical Properties
Ibuprofen acts primarily by inhibiting the activity of cyclooxygenase (COX) enzymes, which are involved in the synthesis of prostaglandins, thromboxanes, and prostacyclin . These biomolecules play key roles in inflammation, pain, and fever. By inhibiting COX enzymes, this compound reduces the production of these compounds, thereby alleviating the associated symptoms .
Cellular Effects
This compound’s effects on cells are largely due to its inhibition of prostaglandin synthesis. Prostaglandins are involved in a variety of cellular processes, including inflammation, pain sensation, and regulation of body temperature . By reducing prostaglandin production, this compound can influence these cellular functions. For example, it can decrease inflammation by reducing the production of inflammatory prostaglandins .
Molecular Mechanism
This compound exerts its effects at the molecular level primarily through its interaction with COX enzymes. It is a non-selective inhibitor of both COX-1 and COX-2 isoforms . This compound binds to the active site of these enzymes, preventing them from converting arachidonic acid into prostaglandins .
Temporal Effects in Laboratory Settings
The effects of this compound can change over time in laboratory settings. For example, prolonged exposure to this compound can lead to the upregulation of COX-2 in certain cell types, potentially reducing the drug’s efficacy . Additionally, this compound has been shown to have a relatively short half-life in the body, which can influence its long-term effects .
Dosage Effects in Animal Models
The effects of this compound in animal models can vary depending on the dosage. At lower doses, this compound primarily acts as an analgesic and antipyretic. At higher doses, it can also exhibit anti-inflammatory effects . High doses of this compound can also lead to gastrointestinal side effects in some animals .
Metabolic Pathways
This compound is metabolized primarily in the liver, where it undergoes oxidation and conjugation reactions to form various metabolites . These metabolites are then excreted in the urine . The enzymes involved in this compound metabolism include several cytochrome P450 isoforms and uridine diphosphate glucuronosyltransferases .
Transport and Distribution
After oral administration, this compound is rapidly absorbed and widely distributed throughout the body . It can cross the blood-brain barrier and placenta, and it is also found in breast milk . This compound is bound to plasma proteins, which can influence its distribution within the body .
Subcellular Localization
As a small, lipophilic molecule, this compound can diffuse across cell membranes and distribute throughout the cell . Its primary site of action is the COX enzymes, which are located in the endoplasmic reticulum and nuclear envelope .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Die Synthese von Ibuprofen erfolgt in mehreren Schritten, beginnend mit Isobutylbenzol. Ein traditionelles Verfahren beinhaltet die folgenden Schritte:
Friedel-Crafts-Acylierung: Isobutylbenzol reagiert mit Essigsäureanhydrid in Gegenwart eines Lewis-Säure-Katalysators, wie Aluminiumchlorid, zu 4-Isobutylacetophenon.
Darzens-Reaktion: Das 4-Isobutylacetophenon unterliegt einer Darzens-Reaktion mit Chloressigsäure unter Bildung eines α,β-Epoxyesters.
Hydrolyse und Decarboxylierung: Der α,β-Epoxyester wird hydrolysiert und decarboxyliert, um 4-Isobutylbenzaldehyd zu liefern.
Oxidation: Das 4-Isobutylbenzaldehyd wird zu 4-Isobutylbenzoesäure oxidiert.
Umlagerung: Die 4-Isobutylbenzoesäure unterliegt einer Hoffmann-Umlagerung unter Bildung von 2-(4-Isobutylphenyl)propansäure, die this compound ist
Industrielle Produktionsverfahren: In industriellen Umgebungen wird this compound häufig mit einem effizienteren und umweltfreundlicheren Verfahren synthetisiert, das als BHC-Verfahren (Boots-Hoechst-Celanese) bekannt ist. Dieses Verfahren reduziert die Synthese auf drei Hauptschritte:
Friedel-Crafts-Acylierung: Ähnlich dem traditionellen Verfahren.
Hydrolyse und Decarboxylierung: Der Zwischenprodukt wird in einem einzigen Schritt hydrolysiert und decarboxyliert.
Umlagerung: Der letzte Umlagerungsschritt zur Herstellung von this compound.
Arten von Reaktionen:
Veresterung: this compound kann Veresterungsreaktionen mit Alkoholen eingehen, um Ester zu bilden, die seine Löslichkeits- und Absorptionsmerkmale verbessern können.
Salzbildung: Die Carbonsäuregruppe von this compound kann mit Basen reagieren, um Salze zu bilden, wodurch seine Bioverfügbarkeit verbessert wird.
Halogenierung: This compound kann Halogenierungsreaktionen eingehen, bei denen Halogene in das Molekül eingeführt werden.
Häufige Reagenzien und Bedingungen:
Veresterung: Beinhaltet typischerweise Alkohole und Säurekatalysatoren.
Salzbildung: Beinhaltet Basen wie Natriumhydroxid oder Kaliumhydroxid.
Halogenierung: Beinhaltet Halogenierungsmittel wie Chlor oder Brom.
Hauptprodukte:
Ester: Aus Veresterungsreaktionen gebildet.
Salze: Aus Reaktionen mit Basen gebildet.
Halogenierte Derivate: Aus Halogenierungsreaktionen gebildet.
Vergleich Mit ähnlichen Verbindungen
- Aspirin (acetylsalicylic acid)
- Naproxen (2-(6-methoxynaphthalen-2-yl)propanoic acid)
- Diclofenac (2-(2,6-dichloranilino)phenylacetic acid)
- Ketoprofen (2-(3-benzoylphenyl)propanoic acid)
Comparison:
- Aspirin: Like ibuprofen, aspirin is a non-selective COX inhibitor but has a higher risk of gastrointestinal side effects.
- Naproxen: Similar to this compound in its anti-inflammatory effects but has a longer half-life, allowing for less frequent dosing.
- Diclofenac: More potent than this compound but associated with higher cardiovascular risks.
- Ketoprofen: Similar in action to this compound but may cause more gastrointestinal discomfort .
This compound stands out due to its balanced efficacy and safety profile, making it one of the most commonly used NSAIDs worldwide.
Eigenschaften
IUPAC Name |
2-[4-(2-methylpropyl)phenyl]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HEFNNWSXXWATRW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)CC1=CC=C(C=C1)C(C)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C13H18O2 | |
| Record name | ibuprofen | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Ibuprofen | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
31121-93-4 (hydrochloride salt), 79261-49-7 (potassium salt) | |
| Record name | Ibuprofen [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015687271 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID5020732 | |
| Record name | Ibuprofen | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5020732 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
206.28 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Readily sol in most org solvents, VERY SOLUBLE IN ALCOHOL, In water, 21 mg/l @ 25 °C, 0.021 mg/mL at 25 °C | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
4.74X10-5 mm Hg @ 25 °C | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The exact mechanism of action of ibuprofen is unknown. However, ibuprofen is considered an NSAID and thus it is a non-selective inhibitor of cyclooxygenase, which is an enzyme involved in prostaglandin (mediators of pain and fever) and thromboxane (stimulators of blood clotting) synthesis via the arachidonic acid pathway. Ibuprofen is a non-selective COX inhibitor and hence, it inhibits the activity of both COX-1 and COX-2. The inhibition of COX-2 activity decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever, and swelling while the inhibition of COX-1 is thought to cause some of the side effects of ibuprofen including GI ulceration., IBUPROFEN AT 25 MG/KG IV INCREASED THE PRIMARY AND TOTAL HEMOSTATIC PLUG FORMATION TIME IN RABBIT EAR CHAMBERS WITH LASER-INDUCED INJURY. THE SAME DOSE INCREASED THE NUMBER OF CUMULATIVE EMBOLI OVER A 10 MINUTE PERIOD AFTER A LASER INJURY TO ARTERIOLES. IN DOGS, DOSES OF 10, 25, AND 50 MG/KG DID NOT ENHANCE THE RELEASE OF (125)I-LABELED FIBRIN DEGRADATION PRODUCTS FROM THE THROMBI AFTER INCUBATION IN PLASMIN, BUT THE LARGEST DOSE SIGNIFICANTLY DECREASED THE THROMBUS WEIGHT 90 AND 180 MINUTES AFTER DRUG ADMINISTRATION. THUS, IBUPROFEN HAD AN INHIBITORY EFFECT ON PLATELET FUNCTION IN VIVO AND IN LARGE DOSES DIMINISHED THE THROMBUS WEIGHT., L-Arginine (L-arg) exhibits multiple biological properties and plays an important role in the regulation of different functions in pathological conditions. Many of these effects could be achieved on this amino acid serving as a substrate for the enzyme nitric oxide synthase (NOS). At the gastrointestinal level, recent reports revealed its protective activities involving a hyperemic response increasing the gastric blood flow. The aim of this study was to characterize the relationship between NOS activity/expression and prostaglandin changes (PGs) in rats gastric mucosa, with L-arg associated resistance to the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen (IBP). The protective effect of oral L-arg (100 mg/kg body wt), administerred together with IBP (100 mg/kg body wt, per os), was evident enough 90 min after drug administration, although a significant protection persisted for more than 6 hr. Pretreatment with N(G)-nitro-L-arginine (L-NNA) (40 mg/kg body wt, intraperitoneally), a competitive inhibitor of constitutive NOS, partly altered the protection afforded by the amino acid. In contrast, no changes could be observed after inducible NOS inhibition [aminoguanidine (AG) 50 mg/Kg body wt, intraperitoneally). L-arg, plus IBP, produced a significant increase of the cyclic GMP (cGMP) response in tissue samples from rat stomach, 90 min and 6 h after drug administration. iNOS activity and mRNA expression were higher in IBP-treated rats, and no differences were observed in inducible responses in the L-arg plus IBP group. No variations in the cNOS activity and expression were found among the different groups of animals assayed. The measurement of mucosal PGE2 content confirmed that biosynthesis of the eicosanoid is maintained by L-arg for over 90 min after IBP, while a total inhibition was observed 6 hr later. The mechanisms of the L-arg protective effect on the damaged induced by IBP could be explained by the different period after drug administration. The early phase is mediated by cyclooxygenase/prostaglandins pathway (COX/PGs) although NO liberated by cNOS and the guanylate cyclase/cGMP pathway could be also relevant. The later phase implicates inhibition of the iNOS/NO response., We previously showed the non-steroidal anti-inflammatory drug (NSAID) ibuprofen suppresses inflammation and amyloid in the APPsw (Tg2576) Tg2576 transgenic mouse. The mechanism for these effects and the impact on behavior are unknown. We now show ibuprofen's effects were not mediated by alterations in amyloid precursor protein (APP) expression or oxidative damage (carbonyls). Six months ibuprofen treatment in Tg+ females caused a decrease in open field behavior (p < 0.05), restoring values similar to Tg- mice. Reduced caspase activation per plaque provided further evidence for a neuroprotective action of ibuprofen.The impact of a shorter 3 month duration ibuprofen trial, beginning at a later age (from 14 to 17 months), was also investigated. Repeated measures ANOVA of Abeta levels (soluble and insoluble) demonstrated a significant ibuprofen treatment effect (p < 0.05). Post-hoc analysis showed that ibuprofen-dependent reductions of both soluble Abeta and Abeta42 were most marked in entorhinal cortex (p < 0.05). Although interleukin-1beta and insoluble Abeta were more effectively reduced with longer treatment, the magnitude of the effect on soluble Abeta was not dependent on treatment duration., Trying to decrease the production of Amyloid beta (Abeta) has been envisaged as a promising approach to prevent neurodegeneration in Alzheimer's disease (AD). A chronic inflammatory reaction with activated microglia cells and astrocytes is a constant feature of AD. The participation of the immune system in the disease process is further documented in several retrospective clinical studies showing an inverse relationship between the prevalence of AD and nonsteroidal anti-inflammatory drug (NSAID) therapy. Previously, we demonstrated that the combination of the proinflammatory cytokines TNFalpha with IFNgamma induces the production of Abeta-42 and Abeta-40 in human neuronal cells. In the present study, the neuronal cell line Sk-n-sh was incubated for 12 h with the cyclooxygenase inhibitor ibuprofen and subsequently stimulated with the cytokines TNFalpha and IFNgamma. Ibuprofen treatment decreased the secretion of total Abeta in the conditioned media of cytokine stimulated cells by 50% and prevented the accumulation of Abeta-42 and Abeta-40 in detergent soluble cell extracts. Viability of neuronal cells measured by detection of apoptosis was neither influenced by ibuprofen nor by cytokine treatment. The reduction in the production of Abeta by ibuprofen was presumably due to a decreased production of betaAPP, which in contrast to the control proteins M2 pyruvate kinase, beta-tubulin and the cytokine inducible ICAM-1 was detected at low concentration in ibuprofen treated cells. The data demonstrate a possible mechanism how ibuprofen may decrease the risk and delay the onset of AD. | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless, crystalline stable solid | |
CAS No. |
15687-27-1 | |
| Record name | Ibuprofen | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=15687-27-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ibuprofen [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015687271 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ibuprofen | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757073 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | ibuprofen | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=256857 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Benzeneacetic acid, .alpha.-methyl-4-(2-methylpropyl)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Ibuprofen | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5020732 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Ibuprofen | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.036.152 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | IBUPROFEN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/WK2XYI10QM | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
75-77.5 ºC, 75-77 °C, 76 °C | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details












Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.