Ibuprofeno
Descripción general
Descripción
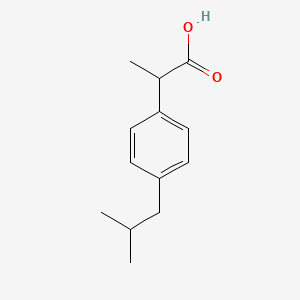
El Ibuprofeno, químicamente conocido como ácido 2-(4-isobutilfenil)propanoico, es un fármaco antiinflamatorio no esteroideo (AINE) ampliamente utilizado. Se emplea principalmente para aliviar el dolor, reducir la fiebre y aliviar la inflamación. Descubierto en 1961 por Stewart Adams y John Nicholson, el this compound se comercializó inicialmente bajo la marca Brufen. Hoy en día, está disponible bajo diversas marcas comerciales, incluyendo Advil, Motrin y Nurofen .
Mecanismo De Acción
El ibuprofeno ejerce sus efectos inhibiendo la enzima ciclooxigenasa (COX), que participa en la síntesis de prostaglandinas. Las prostaglandinas son compuestos lipídicos que median la inflamación, el dolor y la fiebre. Al inhibir la COX, el this compound reduce la producción de prostaglandinas, aliviando así estos síntomas. El this compound es un inhibidor no selectivo de la COX, que afecta a las enzimas COX-1 y COX-2 .
Compuestos Similares:
- Ácido acetilsalicílico (aspirina)
- Naproxeno (ácido 2-(6-metoxinaftalen-2-il)propanoico)
- Diclofenac (ácido 2-(2,6-dicloroanilino)fenilacético)
- Ketoprofeno (ácido 2-(3-benzoilfenil)propanoico)
Comparación:
- Aspirina: Al igual que el this compound, la aspirina es un inhibidor no selectivo de la COX, pero tiene un mayor riesgo de efectos secundarios gastrointestinales.
- Naproxeno: Similar al this compound en sus efectos antiinflamatorios, pero tiene una vida media más larga, lo que permite una dosificación menos frecuente.
- Diclofenac: Más potente que el this compound, pero asociado con mayores riesgos cardiovasculares.
- Ketoprofeno: Similar en acción al this compound, pero puede causar más molestias gastrointestinales .
El this compound destaca por su equilibrado perfil de eficacia y seguridad, convirtiéndolo en uno de los AINE más utilizados en todo el mundo.
Aplicaciones Científicas De Investigación
El ibuprofeno tiene una amplia gama de aplicaciones en la investigación científica:
Análisis Bioquímico
Biochemical Properties
Ibuprofen acts primarily by inhibiting the activity of cyclooxygenase (COX) enzymes, which are involved in the synthesis of prostaglandins, thromboxanes, and prostacyclin . These biomolecules play key roles in inflammation, pain, and fever. By inhibiting COX enzymes, ibuprofen reduces the production of these compounds, thereby alleviating the associated symptoms .
Cellular Effects
Ibuprofen’s effects on cells are largely due to its inhibition of prostaglandin synthesis. Prostaglandins are involved in a variety of cellular processes, including inflammation, pain sensation, and regulation of body temperature . By reducing prostaglandin production, ibuprofen can influence these cellular functions. For example, it can decrease inflammation by reducing the production of inflammatory prostaglandins .
Molecular Mechanism
Ibuprofen exerts its effects at the molecular level primarily through its interaction with COX enzymes. It is a non-selective inhibitor of both COX-1 and COX-2 isoforms . Ibuprofen binds to the active site of these enzymes, preventing them from converting arachidonic acid into prostaglandins .
Temporal Effects in Laboratory Settings
The effects of ibuprofen can change over time in laboratory settings. For example, prolonged exposure to ibuprofen can lead to the upregulation of COX-2 in certain cell types, potentially reducing the drug’s efficacy . Additionally, ibuprofen has been shown to have a relatively short half-life in the body, which can influence its long-term effects .
Dosage Effects in Animal Models
The effects of ibuprofen in animal models can vary depending on the dosage. At lower doses, ibuprofen primarily acts as an analgesic and antipyretic. At higher doses, it can also exhibit anti-inflammatory effects . High doses of ibuprofen can also lead to gastrointestinal side effects in some animals .
Metabolic Pathways
Ibuprofen is metabolized primarily in the liver, where it undergoes oxidation and conjugation reactions to form various metabolites . These metabolites are then excreted in the urine . The enzymes involved in ibuprofen metabolism include several cytochrome P450 isoforms and uridine diphosphate glucuronosyltransferases .
Transport and Distribution
After oral administration, ibuprofen is rapidly absorbed and widely distributed throughout the body . It can cross the blood-brain barrier and placenta, and it is also found in breast milk . Ibuprofen is bound to plasma proteins, which can influence its distribution within the body .
Subcellular Localization
As a small, lipophilic molecule, ibuprofen can diffuse across cell membranes and distribute throughout the cell . Its primary site of action is the COX enzymes, which are located in the endoplasmic reticulum and nuclear envelope .
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción: La síntesis del ibuprofeno implica varios pasos, comenzando con el isobutilbenceno. Un método tradicional incluye los siguientes pasos:
Acilación de Friedel-Crafts: El isobutilbenceno reacciona con anhídrido acético en presencia de un catalizador ácido de Lewis, como el cloruro de aluminio, para formar 4-isobutilacetofenona.
Reacción de Darzens: La 4-isobutilacetofenona se somete a una reacción de Darzens con ácido cloroacético para formar un éster α,β-epóxido.
Hidrólisis y Descarboxilación: El éster α,β-epóxido se hidroliza y descarboxila para producir 4-isobutilbenzaldehído.
Oxidación: El 4-isobutilbenzaldehído se oxida a ácido 4-isobutilbenzoico.
Reordenamiento: El ácido 4-isobutilbenzoico se somete a un reordenamiento de Hofmann para formar ácido 2-(4-isobutilfenil)propanoico, que es this compound
Métodos de Producción Industrial: En entornos industriales, el this compound se sintetiza a menudo utilizando un proceso más eficiente y ecológico conocido como el proceso BHC (Boots-Hoechst-Celanese). Este método reduce la síntesis a tres pasos principales:
Acilación de Friedel-Crafts: Similar al método tradicional.
Hidrólisis y Descarboxilación: El intermedio se hidroliza y descarboxila en un solo paso.
Reordenamiento: El paso final de reordenamiento para producir this compound.
Tipos de Reacciones:
Esterificación: El this compound puede sufrir reacciones de esterificación con alcoholes para formar ésteres, que pueden mejorar sus características de solubilidad y absorción.
Formación de Sales: El grupo ácido carboxílico del this compound puede reaccionar con bases para formar sales, mejorando su biodisponibilidad.
Halogenación: El this compound puede sufrir reacciones de halogenación, donde se introducen halógenos en la molécula.
Reactivos y Condiciones Comunes:
Esterificación: Suele implicar alcoholes y catalizadores ácidos.
Formación de Sales: Implica bases como hidróxido de sodio o hidróxido de potasio.
Halogenación: Implica agentes halogenantes como cloro o bromo.
Productos Principales:
Ésteres: Formado a partir de reacciones de esterificación.
Sales: Formado a partir de reacciones con bases.
Derivados Halogenados: Formado a partir de reacciones de halogenación.
Comparación Con Compuestos Similares
- Aspirin (acetylsalicylic acid)
- Naproxen (2-(6-methoxynaphthalen-2-yl)propanoic acid)
- Diclofenac (2-(2,6-dichloranilino)phenylacetic acid)
- Ketoprofen (2-(3-benzoylphenyl)propanoic acid)
Comparison:
- Aspirin: Like ibuprofen, aspirin is a non-selective COX inhibitor but has a higher risk of gastrointestinal side effects.
- Naproxen: Similar to ibuprofen in its anti-inflammatory effects but has a longer half-life, allowing for less frequent dosing.
- Diclofenac: More potent than ibuprofen but associated with higher cardiovascular risks.
- Ketoprofen: Similar in action to ibuprofen but may cause more gastrointestinal discomfort .
Ibuprofen stands out due to its balanced efficacy and safety profile, making it one of the most commonly used NSAIDs worldwide.
Propiedades
IUPAC Name |
2-[4-(2-methylpropyl)phenyl]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HEFNNWSXXWATRW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)CC1=CC=C(C=C1)C(C)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C13H18O2 | |
| Record name | ibuprofen | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Ibuprofen | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
31121-93-4 (hydrochloride salt), 79261-49-7 (potassium salt) | |
| Record name | Ibuprofen [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015687271 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID5020732 | |
| Record name | Ibuprofen | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5020732 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
206.28 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Readily sol in most org solvents, VERY SOLUBLE IN ALCOHOL, In water, 21 mg/l @ 25 °C, 0.021 mg/mL at 25 °C | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
4.74X10-5 mm Hg @ 25 °C | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The exact mechanism of action of ibuprofen is unknown. However, ibuprofen is considered an NSAID and thus it is a non-selective inhibitor of cyclooxygenase, which is an enzyme involved in prostaglandin (mediators of pain and fever) and thromboxane (stimulators of blood clotting) synthesis via the arachidonic acid pathway. Ibuprofen is a non-selective COX inhibitor and hence, it inhibits the activity of both COX-1 and COX-2. The inhibition of COX-2 activity decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever, and swelling while the inhibition of COX-1 is thought to cause some of the side effects of ibuprofen including GI ulceration., IBUPROFEN AT 25 MG/KG IV INCREASED THE PRIMARY AND TOTAL HEMOSTATIC PLUG FORMATION TIME IN RABBIT EAR CHAMBERS WITH LASER-INDUCED INJURY. THE SAME DOSE INCREASED THE NUMBER OF CUMULATIVE EMBOLI OVER A 10 MINUTE PERIOD AFTER A LASER INJURY TO ARTERIOLES. IN DOGS, DOSES OF 10, 25, AND 50 MG/KG DID NOT ENHANCE THE RELEASE OF (125)I-LABELED FIBRIN DEGRADATION PRODUCTS FROM THE THROMBI AFTER INCUBATION IN PLASMIN, BUT THE LARGEST DOSE SIGNIFICANTLY DECREASED THE THROMBUS WEIGHT 90 AND 180 MINUTES AFTER DRUG ADMINISTRATION. THUS, IBUPROFEN HAD AN INHIBITORY EFFECT ON PLATELET FUNCTION IN VIVO AND IN LARGE DOSES DIMINISHED THE THROMBUS WEIGHT., L-Arginine (L-arg) exhibits multiple biological properties and plays an important role in the regulation of different functions in pathological conditions. Many of these effects could be achieved on this amino acid serving as a substrate for the enzyme nitric oxide synthase (NOS). At the gastrointestinal level, recent reports revealed its protective activities involving a hyperemic response increasing the gastric blood flow. The aim of this study was to characterize the relationship between NOS activity/expression and prostaglandin changes (PGs) in rats gastric mucosa, with L-arg associated resistance to the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen (IBP). The protective effect of oral L-arg (100 mg/kg body wt), administerred together with IBP (100 mg/kg body wt, per os), was evident enough 90 min after drug administration, although a significant protection persisted for more than 6 hr. Pretreatment with N(G)-nitro-L-arginine (L-NNA) (40 mg/kg body wt, intraperitoneally), a competitive inhibitor of constitutive NOS, partly altered the protection afforded by the amino acid. In contrast, no changes could be observed after inducible NOS inhibition [aminoguanidine (AG) 50 mg/Kg body wt, intraperitoneally). L-arg, plus IBP, produced a significant increase of the cyclic GMP (cGMP) response in tissue samples from rat stomach, 90 min and 6 h after drug administration. iNOS activity and mRNA expression were higher in IBP-treated rats, and no differences were observed in inducible responses in the L-arg plus IBP group. No variations in the cNOS activity and expression were found among the different groups of animals assayed. The measurement of mucosal PGE2 content confirmed that biosynthesis of the eicosanoid is maintained by L-arg for over 90 min after IBP, while a total inhibition was observed 6 hr later. The mechanisms of the L-arg protective effect on the damaged induced by IBP could be explained by the different period after drug administration. The early phase is mediated by cyclooxygenase/prostaglandins pathway (COX/PGs) although NO liberated by cNOS and the guanylate cyclase/cGMP pathway could be also relevant. The later phase implicates inhibition of the iNOS/NO response., We previously showed the non-steroidal anti-inflammatory drug (NSAID) ibuprofen suppresses inflammation and amyloid in the APPsw (Tg2576) Tg2576 transgenic mouse. The mechanism for these effects and the impact on behavior are unknown. We now show ibuprofen's effects were not mediated by alterations in amyloid precursor protein (APP) expression or oxidative damage (carbonyls). Six months ibuprofen treatment in Tg+ females caused a decrease in open field behavior (p < 0.05), restoring values similar to Tg- mice. Reduced caspase activation per plaque provided further evidence for a neuroprotective action of ibuprofen.The impact of a shorter 3 month duration ibuprofen trial, beginning at a later age (from 14 to 17 months), was also investigated. Repeated measures ANOVA of Abeta levels (soluble and insoluble) demonstrated a significant ibuprofen treatment effect (p < 0.05). Post-hoc analysis showed that ibuprofen-dependent reductions of both soluble Abeta and Abeta42 were most marked in entorhinal cortex (p < 0.05). Although interleukin-1beta and insoluble Abeta were more effectively reduced with longer treatment, the magnitude of the effect on soluble Abeta was not dependent on treatment duration., Trying to decrease the production of Amyloid beta (Abeta) has been envisaged as a promising approach to prevent neurodegeneration in Alzheimer's disease (AD). A chronic inflammatory reaction with activated microglia cells and astrocytes is a constant feature of AD. The participation of the immune system in the disease process is further documented in several retrospective clinical studies showing an inverse relationship between the prevalence of AD and nonsteroidal anti-inflammatory drug (NSAID) therapy. Previously, we demonstrated that the combination of the proinflammatory cytokines TNFalpha with IFNgamma induces the production of Abeta-42 and Abeta-40 in human neuronal cells. In the present study, the neuronal cell line Sk-n-sh was incubated for 12 h with the cyclooxygenase inhibitor ibuprofen and subsequently stimulated with the cytokines TNFalpha and IFNgamma. Ibuprofen treatment decreased the secretion of total Abeta in the conditioned media of cytokine stimulated cells by 50% and prevented the accumulation of Abeta-42 and Abeta-40 in detergent soluble cell extracts. Viability of neuronal cells measured by detection of apoptosis was neither influenced by ibuprofen nor by cytokine treatment. The reduction in the production of Abeta by ibuprofen was presumably due to a decreased production of betaAPP, which in contrast to the control proteins M2 pyruvate kinase, beta-tubulin and the cytokine inducible ICAM-1 was detected at low concentration in ibuprofen treated cells. The data demonstrate a possible mechanism how ibuprofen may decrease the risk and delay the onset of AD. | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless, crystalline stable solid | |
CAS No. |
15687-27-1 | |
| Record name | Ibuprofen | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=15687-27-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ibuprofen [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015687271 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ibuprofen | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757073 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | ibuprofen | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=256857 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Benzeneacetic acid, .alpha.-methyl-4-(2-methylpropyl)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Ibuprofen | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5020732 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Ibuprofen | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.036.152 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | IBUPROFEN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/WK2XYI10QM | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
75-77.5 ºC, 75-77 °C, 76 °C | |
| Record name | Ibuprofen | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01050 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBUPROFEN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3099 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ibuprofen | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001925 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details












Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.