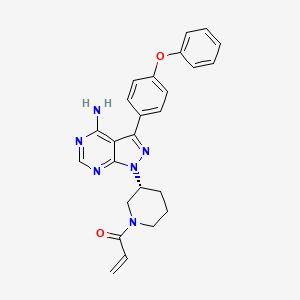
Ibrutinib
Übersicht
Beschreibung
Ibrutinib ist ein niedermolekulares Medikament, das als irreversibler Inhibitor der Bruton-Tyrosinkinase (BTK) wirkt. Es wird hauptsächlich zur Behandlung verschiedener B-Zell-Malignome eingesetzt, darunter chronische lymphatische Leukämie, Mantelzelllymphom und Waldenström-Makroglobulinämie . This compound wurde erstmals im November 2013 von der US-amerikanischen Food and Drug Administration zugelassen und hat sich seitdem zu einem wichtigen Bestandteil der Behandlung von B-Zell-Krebsarten entwickelt .
Wirkmechanismus
Target of Action
Ibrutinib primarily targets Bruton’s tyrosine kinase (BTK) . BTK is a crucial component of multiple signaling pathways that regulate B cell and myeloid cell proliferation, survival, and functions . It plays a central role in the B-cell receptor signaling pathway , making it a promising therapeutic target for various B-cell malignancies and inflammatory diseases .
Mode of Action
This compound acts as an irreversible potent inhibitor of BTK . It forms a covalent bond with a cysteine residue (C481) in the BTK active site , leading to sustained inhibition of BTK enzymatic activity . This inhibition disrupts the growth signal by targeting BTK in patients with B-cell malignancies .
Biochemical Pathways
This compound’s action affects various signaling pathways, including BCR signaling, chemokine receptor signaling, TLR signaling, and FcR signaling . By inhibiting BTK, this compound impairs B-cell receptor (BCR) signaling pathway and inhibits B-cell proliferation, survival, and migration .
Pharmacokinetics
This compound is orally bioavailable . It has been demonstrated to have high inter-individual variability in its pharmacokinetic profiles . Smoking was identified as a covariate that affects the observed this compound concentrations. Smokers are expected to have around a 40% reduction in steady-state this compound concentrations compared to non-smokers .
Result of Action
The molecular and cellular effects of this compound’s action are multifaceted. It reduces B-cell proliferation by inhibiting BTK . It also directly impacts the homeostasis, phenotype, and function of many other cell subsets of the immune system . After the first year of this compound treatment, the pathologically high frequencies of exhausted or chronically activated effector/memory CD4 and CD8 T cells as well as Tregs are reduced, while naive T cells are preserved and T cell receptor (TCR) repertoire diversity is significantly increased .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, smoking status has been identified as a significant factor associated with this compound clearance . Moreover, the microenvironment typical of chronic lymphocytic leukemia (CLL) can modulate the action of this compound . The over-activation of the BCR can be due to a microenvironmental antigen-mediated stimulation or be the result of a constitutive activation phenomenon .
Wissenschaftliche Forschungsanwendungen
Ibrutinib hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Wird als Modellverbindung für die Untersuchung kovalenter Inhibitoren und ihrer Wechselwirkungen mit Zielproteinen verwendet.
Biologie: Wird auf seine Auswirkungen auf die B-Zell-Rezeptor-Signalgebung und seine Rolle in der Immunzellfunktion untersucht.
Medizin: Wird in klinischen Studien für verschiedene B-Zell-Malignome und andere Krebserkrankungen ausgiebig verwendet. .
5. Wirkmechanismus
This compound entfaltet seine Wirkung, indem es irreversibel an den Cystein-481-Rest im aktiven Zentrum der Bruton-Tyrosinkinase bindet. Diese Bindung hemmt die Kinaseaktivität von BTK und blockiert so den B-Zell-Rezeptor-Signalweg. Diese Hemmung führt zu einer verminderten Proliferation und Überlebensfähigkeit von malignen B-Zellen . Darüber hinaus beeinflusst this compound andere Kinasen wie C-terminale Src-Kinasen, was zu seinen therapeutischen Wirkungen beiträgt .
Ähnliche Verbindungen:
Acalabrutinib: Ein weiterer BTK-Inhibitor mit einem ähnlichen Wirkmechanismus, aber unterschiedlichen pharmakokinetischen Eigenschaften.
Zanubrutinib: Ein BTK-Inhibitor der zweiten Generation mit verbesserter Selektivität und reduzierten Off-Target-Effekten.
Tirabrutinib: Ein BTK-Inhibitor, der zur Behandlung von B-Zell-Malignomen eingesetzt wird, mit dem Schwerpunkt auf der Reduzierung von Nebenwirkungen
Vergleich: this compound ist einzigartig in seiner Fähigkeit, irreversibel an BTK zu binden, was eine anhaltende Hemmung des B-Zell-Rezeptor-Signalwegs ermöglicht. Im Vergleich zu Acalabrutinib und Zanubrutinib hat this compound ein breiteres Spektrum an Kinase-Zielstrukturen, was zu einem breiteren Spektrum an therapeutischen Wirkungen, aber auch zu einem höheren Potenzial für Nebenwirkungen führen kann . Acalabrutinib und Zanubrutinib sollen eine selektivere Hemmung bieten, was die Nebenwirkungen möglicherweise reduziert und gleichzeitig die Wirksamkeit erhalten .
Biochemische Analyse
Biochemical Properties
Ibrutinib forms a covalent bond with a cysteine residue in the active site of BTK (Cys481), leading to its inhibition . The inhibition of BTK plays a role in the B-cell receptor signaling and thus, the presence of this compound prevents the phosphorylation of downstream substrates such as PLC-γ .
Cellular Effects
This compound blocks the proteins (kinases) from sending signals to the cancer cells to grow. Blocking the signals causes the cancer cells to die. This may help to stop or slow down the cancer growing . This compound also has complex immunomodulatory effects on various non-B immune cell subsets by inhibiting BTK-dependent signaling pathways of specific immune receptors .
Molecular Mechanism
This compound is a potent, irreversible inhibitor of Bruton’s tyrosine kinase (BTK). The acrylamide group of this compound forms a covalent bond with the cysteine residue C481 in the BTK active site, leading to sustained inhibition of BTK enzymatic activity . This compound also binds to C-terminal Src Kinases, inhibiting the kinase from promoting cell differentiation and growth .
Temporal Effects in Laboratory Settings
This compound-related adverse events are common and have resulted in discontinuation of therapy in >20% of patients taking the drug in a real-world setting . A pilot trial evaluated stepwise reduction of this compound dose in patients with CLL from 420 to 280 to 140 mg/d over three 28-day cycles .
Dosage Effects in Animal Models
In rodent glioma models, this compound (25 mg/kg) combined with doxil prolonged median survival . In another study, this compound (10 mg/kg) protected against poly I:C-induced (5 mg/kg) and LPS-induced (5 mg/kg) lung inflammation .
Metabolic Pathways
The metabolism of this compound is mainly performed by CYP3A5 and CYP3A4, and to a minor extent, it is seen to be performed by CYP2D6 .
Transport and Distribution
This compound is rapidly absorbed after oral administration and it presents a Cmax, tmax and AUC of approximately 35 ng/ml, 1-2 hour and 953 mg.h/ml respectively . The volume of distribution at steady-state of this compound is approximately 10,000 L .
Subcellular Localization
Resistance to this compound can be conferred via aberrant nuclear/cytoplasmic subcellular localization of FOXO3a . This compound induces nuclear retention of IκB that was accompanied by the reduction of DNA-binding activity of NFκB, suggesting that NFκB is trapped in an inhibitory complex .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Die Synthese von Ibrutinib umfasst mehrere wichtige Schritte:
Kondensation und Methoxylierung: 4-Benzyloxybenzoylchlorid wird mit Malononitril und Dimethylsulfat umgesetzt, um 4-Benzyloxyphenyl(methoxy)vinylidenedicyanomethan zu erzeugen.
Pyrazol-Cyclisierung: Die Zwischenverbindung wird dann einer Pyrazol-Cyclisierung mit 1-(3R-Hydrazino-1-piperidino)-2-propen-1-keton unterzogen, um 1-[(3R)-[3-(4-Benzyloxyphenyl)-4-nitril-5-amino-1H-pyrazol]-1-piperidino]-2-propen-1-keton zu bilden.
Pyrimidin-Cyclisierung: Schließlich erfolgt die Pyrimidin-Cyclisierung, um this compound zu erhalten
Industrielle Produktionsmethoden: Die industrielle Produktion von this compound erfolgt typischerweise in großem Maßstab unter Verwendung der oben genannten Schritte, wobei die Ausbeute und Reinheit optimiert werden. Der Prozess kann zusätzliche Reinigungsschritte wie Umkristallisation und Chromatographie umfassen, um sicherzustellen, dass das Endprodukt den pharmazeutischen Standards entspricht .
Arten von Reaktionen:
Oxidation: this compound kann Oxidationsreaktionen eingehen, insbesondere an der Phenoxyphenyl-Gruppierung.
Reduktion: Reduktionsreaktionen können auf die Nitrilgruppe im Molekül abzielen.
Substitution: Nucleophile Substitutionsreaktionen können am Piperidinring auftreten.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Oxidationsmittel sind Wasserstoffperoxid und Kaliumpermanganat.
Reduktion: Reduktionsmittel wie Lithiumaluminiumhydrid und Natriumborhydrid werden verwendet.
Substitution: Die Bedingungen umfassen häufig die Verwendung starker Nucleophile wie Natriumhydrid oder Kalium-tert-butoxid
Hauptprodukte: Die bei diesen Reaktionen entstehenden Hauptprodukte hängen von den jeweiligen Bedingungen und verwendeten Reagenzien ab. So kann die Oxidation zu hydroxylierten Derivaten führen, während die Reduktion Amin-Derivate erzeugen kann .
Vergleich Mit ähnlichen Verbindungen
Acalabrutinib: Another BTK inhibitor with a similar mechanism of action but different pharmacokinetic properties.
Zanubrutinib: A second-generation BTK inhibitor with improved selectivity and reduced off-target effects.
Tirabrutinib: A BTK inhibitor used in the treatment of B-cell malignancies with a focus on reducing adverse effects
Comparison: Ibrutinib is unique in its ability to irreversibly bind to BTK, providing sustained inhibition of the B-cell receptor pathway. Compared to acalabrutinib and zanubrutinib, this compound has a broader range of kinase targets, which can lead to a wider spectrum of therapeutic effects but also a higher potential for side effects . Acalabrutinib and zanubrutinib are designed to offer more selective inhibition, potentially reducing adverse effects while maintaining efficacy .
Eigenschaften
IUPAC Name |
1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XYFPWWZEPKGCCK-GOSISDBHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C=CC(=O)N1CCCC(C1)N2C3=NC=NC(=C3C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C=CC(=O)N1CCC[C@H](C1)N2C3=NC=NC(=C3C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H24N6O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60893450 | |
| Record name | Ibrutinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60893450 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
440.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble in water, Freely soluble in dimethyl sulfoxide; soluble in methanol | |
| Record name | Ibrutinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09053 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBRUTINIB | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8260 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Ibrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK). It forms a covalent bond with a cysteine residue in the active site of BTK (Cys481), leading to its inhibition. The inhibition of BTK plays a role in the B-cell receptor signaling and thus, the presence of ibrutinib prevents the phosphorylation of downstream substrates such as PLC-γ., Ibrutinib is a novel oral tyrosine kinase inhibitor that irreversibly binds and inhibits tyrosine-protein kinase BTK (Bruton tyrosine kinase). BTK has been found to be important in the function of B-cell receptor signaling and therefore in the maintenance and expansion of various B-cell malignancies including chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Targeting BTK with ibrutinib has been found to be an effective strategy in treating these malignancies. Phase I clinical testing in non-Hodgkin's lymphomas and CLL showed that the drug was extremely well tolerated with no major dose-limiting toxicities and a 54% overall response rate. Subsequently, two phase Ib/II studies were performed on patients with CLL, one in relapsed/refractory CLL and one in previously untreated elderly patients with CLL. Both of these studies continued to show good tolerability of the drug and an overall response rate of about 71% with extended duration of response. Another phase II study using ibrutinib in relapsed/refractory MCL was conducted and also showed that it was well tolerated with an overall response rate of 68% and extended duration of response. Due to these results, the U.S. Food and Drug Administration granted accelerated approval for ibrutinib in November 2013 for patients with MCL who had received at least one prior therapy and in February 2014 for patients with CLL who had received at least one prior therapy. This review will discuss the preclinical pharmacology, pharmacokinetics and clinical efficacy to date of ibrutinib in the treatment of CLL and MCL., ... In this study we report for the first time that ibrutinib is cytotoxic to malignant plasma cells from patients with multiple myeloma (MM) and furthermore that treatment with ibrutinib significantly augments the cytotoxic activity of bortezomib and lenalidomide chemotherapies. We describe that the cytotoxicity of ibrutinib in MM is mediated via an inhibitory effect on the nuclear factor-(k)B (NF-(k)B) pathway. Specifically, ibrutinib blocks the phosphorylation of serine-536 of the p65 subunit of NF-(k)B, preventing its nuclear translocation, resulting in down-regulation of anti-apoptotic proteins Bcl-xL, FLIP(L) and survivin and culminating in caspase-mediated apoptosis within the malignant plasma cells...., Mantle cell lymphoma (MCL) is an aggressive B-cell malignancy that characteristically shows overexpression of cyclin-D1 due to an alteration in the t(11;14)(q13;q32) chromosomal region. Although there are some promising treatment modalities, great majority of patients with this disease remain incurable. The B-cell antigen receptor (BCR) signaling plays a crucial role in B-cell biology and lymphomagenesis. Bruton tyrosine kinase (BTK) has been identified as a key component of the BCR signaling pathway. Evidence suggests that the blockade of BTK activity by potent pharmacologic inhibitors attenuates BCR signaling and induces cell death. Notably, the expression levels and the role of BTK in MCL survival are still elusive. Here, we demonstrated a moderate to strong BTK expression in all MCL cases (n=19) compared to benign lymphoid tissues. Treatment of MCL cell lines (Mino or Jeko-1) with a potent BTK pharmacologic inhibitor, Ibrutinib, decreased phospho-BTK-Tyr(223) expression. Consistent with this observation, Ibrutinib inhibited the viability of both Mino and JeKo-1 cells in concentration- and time-dependent manners. Ibrutinib also induced a concentration-dependent apoptosis in both cell lines. Consistently, Ibrutinib treatment decreased the levels of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1 protein. These findings suggest that BTK signaling plays a critical role in MCL cell survival, and the targeting of BTK could represent a promising therapeutic modality for aggressive lymphoma., Ibrutinib is a small-molecule inhibitor of Bruton's tyrosine kinase (BTK). Ibrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. BTK's role in signaling through the B-cell surface receptors results in activation of pathways necessary for B-cell trafficking, chemotaxis, and adhesion. Nonclinical studies show that ibrutinib inhibits malignant B-cell proliferation and survival in vivo as well as cell migration and substrate adhesion in vitro. | |
| Record name | Ibrutinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09053 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | IBRUTINIB | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8260 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to off-white solid | |
CAS No. |
936563-96-1 | |
| Record name | Ibrutinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=936563-96-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ibrutinib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0936563961 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ibrutinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09053 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ibrutinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60893450 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | IBRUTINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1X70OSD4VX | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | IBRUTINIB | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8260 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
149-158ºC | |
| Record name | Ibrutinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09053 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![12,14-dimethyl-9-(5-methylfuran-2-yl)-17-phenyl-1,8,12,14-tetrazatetracyclo[8.7.0.02,7.011,16]heptadeca-2,4,6,10,16-pentaene-13,15-dione](/img/structure/B1684367.png)
![N-[(1R,2R)-1-(2,3-dihydro-1,4-benzodioxin-6-yl)-1-hydroxy-3-pyrrolidin-1-ylpropan-2-yl]nonanamide](/img/structure/B1684369.png)