Diazoxide
Übersicht
Beschreibung
Diazoxide is a non-diuretic benzothiadiazine derivative that is primarily used to manage hypoglycemia due to hyperinsulinism. It is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and lacks chloriuretic or natriuretic activity . This compound is known for its ability to activate ATP-sensitive potassium channels, which helps in inhibiting insulin release .
Wirkmechanismus
Target of Action
Diazoxide primarily targets the ATP-sensitive potassium channels, specifically the sulfonylurea receptor-1 subunit in the ATP-sensitive K+ (KATP) channel . These channels are present on the insulin-producing beta cells of the pancreas .
Mode of Action
This compound works by activating the ATP-sensitive potassium channels . This activation leads to the hyperpolarization of the cell membrane, which in turn decreases calcium influx and subsequently reduces the release of insulin . This mechanism of action is the mirror opposite of that of sulfonylureas, a class of medications used to increase insulin release in type 2 diabetics .
Biochemical Pathways
This compound’s action on the ATP-sensitive potassium channels leads to a series of downstream effects. The hyperpolarization of the cell membrane inhibits the secretion of insulin by the pancreas . This results in an increase in blood glucose levels . This compound also exhibits hypotensive activity and reduces arteriolar smooth muscle and vascular resistance .
Pharmacokinetics
This compound is readily and extensively absorbed. Peak blood concentrations are attained in 4 hours following oral administration . The drug is metabolized in the liver through oxidation and sulfate conjugation . The elimination half-life of this compound is between 21-45 hours, and it is excreted via the kidneys .
Result of Action
The primary result of this compound’s action is the inhibition of insulin release, leading to an increase in blood glucose levels . This makes this compound effective in the treatment of hyperinsulinaemic hypoglycemia . Additionally, this compound’s hypotensive activity can be used to treat hypertensive emergencies .
Action Environment
The efficacy and stability of this compound can be influenced by various environmental factors. For instance, the drug’s absorption, distribution, metabolism, and excretion can be affected by factors such as the patient’s age, liver function, and renal function . Furthermore, this compound’s effectiveness can be reduced in non-insulin dependent diabetic patients due to its mechanism of action .
Biochemische Analyse
Biochemical Properties
Diazoxide activates ATP-sensitive potassium channels . This activation leads to the hyperpolarization of cell membranes, which in turn inhibits the release of insulin . This biochemical property makes this compound particularly useful in the treatment of hyperinsulinemic hypoglycemia .
Cellular Effects
This compound has a significant impact on various types of cells and cellular processes. It influences cell function by inhibiting insulin release . This inhibition can affect cell signaling pathways, gene expression, and cellular metabolism . This compound also exhibits hypotensive activity and reduces arteriolar smooth muscle and vascular resistance .
Molecular Mechanism
This compound works by binding to the sulfonylurea receptor (SUR) subunit of the ATP-sensitive potassium channel (K ATP) channel on the membrane of pancreatic beta‐cells . This binding promotes a potassium efflux from beta-cells, leading to the inhibition of insulin release .
Temporal Effects in Laboratory Settings
The effects of this compound can change over time in laboratory settings. The plasma half-life of this compound varies from 9.5 to 24 hours in children with normal renal function and from 20 to 72 hours in adults with normal renal function . This indicates that the product’s stability and degradation, as well as any long-term effects on cellular function, need to be considered in both in vitro and in vivo studies .
Metabolic Pathways
This compound is metabolized in the liver through oxidation of the 3-methyl group, producing hydroxymethyl (MI) and carboxy (M2) derivatives . The MI derivatives undergo subsequent sulphate conjugation . It is estimated that, in subjects with normal renal function, 54-60% of this compound is metabolized .
Subcellular Localization
This compound is known to affect mitochondrial bioenergetics by opening the mKATP channel This suggests that this compound may be localized in the mitochondria of cells
Vorbereitungsmethoden
Die Herstellung von Diazoxid beinhaltet mehrere synthetische Routen. Eine gängige Methode beinhaltet die Reaktion von o-Aminobenzolsulfonamid mit N-Chlorsuccinimid in einem Chlorsolvens, um 2-Amino-5-Chlorbenzolsulfonamid zu erhalten. Dieses Zwischenprodukt wird dann mit einem Imidazolsalz und einem Amid-Lösungsmittel vermischt und anschließend erhitzt, um Diazoxid zu erhalten . Dieses Verfahren vermeidet die Verwendung stark korrosiver und giftiger Reagenzien, wodurch es für die industrielle Produktion besser geeignet ist .
Analyse Chemischer Reaktionen
Diazoxid unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: Diazoxid kann unter bestimmten Bedingungen oxidiert werden, obwohl detaillierte Reaktionswege weniger häufig diskutiert werden.
Reduktion: Reduktionsreaktionen mit Diazoxid werden nicht häufig berichtet.
Substitution: Diazoxid kann an Substitutionsreaktionen teilnehmen, insbesondere unter Einbeziehung seiner funktionellen Gruppen. Gängige Reagenzien, die in diesen Reaktionen verwendet werden, umfassen N-Chlorsuccinimid und Imidazolsalze
Wissenschaftliche Forschungsanwendungen
Diazoxid hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Wird in verschiedenen synthetischen Wegen und als Reagenz in Mehrkomponentenreaktionen verwendet.
Biologie: Wird auf seine Auswirkungen auf zelluläre Kaliumkanäle und seine Rolle bei der Modulation zellulärer Aktivitäten untersucht.
Medizin: Wird hauptsächlich zur Behandlung von hyperinsulinämischer Hypoglykämie eingesetzt, indem die Insulin-Freisetzung gehemmt wird.
Industrie: Wird bei der Herstellung von Pharmazeutika und als Forschungswerkzeug in der Arzneimittelentwicklung eingesetzt.
5. Wirkmechanismus
Diazoxid wirkt, indem es ATP-sensitive Kaliumkanäle in den Betazellen der Bauchspeicheldrüse aktiviert. Diese Aktivierung führt zur Öffnung der Kaliumkanäle, was eine Hyperpolarisierung der Zellmembran verursacht. Dies führt zu einer Abnahme des Kalziumeinstroms, der die Freisetzung von Insulin hemmt . Dieser Mechanismus ist das Gegenteil von dem von Sulfonylharnstoffen, die die Insulin-Freisetzung erhöhen .
Vergleich Mit ähnlichen Verbindungen
Diazoxid ist chemisch ähnlich wie Thiazid-Diuretika, unterscheidet sich jedoch in seiner fehlenden diuretischen Aktivität. Ähnliche Verbindungen umfassen:
Thiazid-Diuretika: Wie Hydrochlorothiazid, die zur Behandlung von Bluthochdruck und Ödemen eingesetzt werden, aber andere Wirkmechanismen haben.
Sulfonylharnstoffe: Wie Glibenclamid, die die Insulin-Freisetzung erhöhen und zur Behandlung von Typ-2-Diabetes eingesetzt werden. Die einzigartige Fähigkeit von Diazoxid, die Insulin-Freisetzung zu hemmen und gleichzeitig keine diuretische Aktivität zu besitzen, hebt es von diesen ähnlichen Verbindungen ab.
Eigenschaften
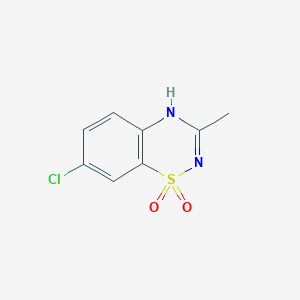
IUPAC Name |
7-chloro-3-methyl-4H-1λ6,2,4-benzothiadiazine 1,1-dioxide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H7ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-4H,1H3,(H,10,11) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GDLBFKVLRPITMI-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NS(=O)(=O)C2=C(N1)C=CC(=C2)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H7ClN2O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7022914 | |
| Record name | Diazoxide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022914 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
230.67 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
5.52e-01 g/L | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane. Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity. It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance. | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
364-98-7 | |
| Record name | Diazoxide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=364-98-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diazoxide [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000364987 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759574 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=76130 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=64198 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Diazoxide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022914 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diazoxide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.006.063 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DIAZOXIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/O5CB12L4FN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
330.5 °C | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary target of diazoxide, and how does this interaction affect pancreatic β-cells?
A1: this compound primarily targets mitochondrial ATP-sensitive potassium (mitoKATP) channels [, , , ]. By binding to and opening these channels, this compound induces hyperpolarization of the β-cell membrane, effectively inhibiting insulin secretion [, , , , ]. This mechanism is thought to involve increased potassium permeability, ultimately reducing calcium influx and blocking insulin release [, , , ].
Q2: Beyond mitoKATP channels, does this compound interact with other targets to influence cellular processes?
A2: Research suggests that this compound might directly interact with mitochondrial F0F1 ATP synthase, promoting the binding of the inhibitor protein IF(1) and reversibly inhibiting ATP hydrolysis []. This interaction could contribute to the cardioprotective effects observed with this compound treatment [, , ].
Q3: this compound is known to influence calcium signaling in various cell types. How does this occur?
A3: this compound can modulate calcium signaling through multiple pathways. In rat ventricular myocytes, this compound has been shown to modulate the opening of the mitochondrial permeability transition pore, leading to an increase in calcium transients independently of changes in mitochondrial membrane potential []. In endothelial cells, this compound can increase intracellular calcium levels through both mitochondrial reactive oxygen species-dependent and -independent mechanisms, leading to activation of endothelial nitric oxide synthase (eNOS) and subsequent vasodilation [].
Q4: What is the molecular formula and weight of this compound?
A4: this compound has the molecular formula C8H7ClN2O3S and a molecular weight of 230.66 g/mol.
Q5: How do structural modifications of this compound impact its activity and selectivity for KATP channels?
A5: While the provided papers don't directly compare different this compound analogs, they highlight the importance of specific structural features. For instance, the presence of the sulfonylurea moiety is crucial for its interaction with KATP channels []. Further research exploring SAR could reveal modifications that enhance selectivity for mitoKATP channels over other subtypes.
Q6: What is the primary route of administration for this compound, and what is known about its pharmacokinetic profile?
A7: this compound can be administered intravenously or orally [, ]. It exhibits rapid but transient effects on plasma insulin and glucose levels, suggesting a short half-life []. One study observed that this compound crosses the placental barrier and is present in amniotic fluid and urine during fetal exposure [].
Q7: What are the documented effects of this compound on blood glucose and insulin levels in animal models?
A8: this compound consistently induces hyperglycemia in various animal models, including rats and mice [, ]. This effect is attributed to its inhibitory action on insulin secretion from pancreatic β-cells [, , ]. Notably, the magnitude of hyperglycemia can be modulated by calcium receptor activity [].
Q8: What is the clinical significance of this compound responsiveness in patients with congenital hyperinsulinism (CHI)?
A9: this compound responsiveness is a crucial factor in the management of CHI [, , ]. Patients with KATP-hyperinsulinism, particularly those with mutations in the ABCC8 or KCNJ11 genes, often exhibit this compound unresponsiveness []. This characteristic can guide treatment decisions, as this compound may be less effective in these cases, necessitating alternative therapies like octreotide or surgery [, ].
Q9: Have any adverse effects been reported in association with this compound treatment?
A10: While the provided papers don't extensively discuss adverse effects, some studies mention alopecia and hypertrichosis lanuginosa as potential side effects observed in infants exposed to this compound in utero []. Additionally, one study reports a case of this compound-induced neutropenia after prolonged treatment []. These findings highlight the importance of monitoring for potential side effects during this compound therapy.
Q10: Are there known mechanisms of resistance to this compound, particularly in the context of CHI?
A11: this compound unresponsiveness is a significant challenge in CHI management. Mutations in the ABCC8 and KCNJ11 genes, encoding subunits of the KATP channel, are strongly associated with this compound resistance [, ]. This suggests that these mutations disrupt the drug's ability to bind to and activate the channel, rendering it ineffective in suppressing insulin secretion.
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![5-(4'-Methyl-[1,1'-biphenyl]-2-yl)-1-trityl-1H-tetrazole](/img/structure/B193154.png)