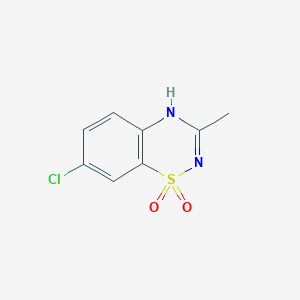
Diazoxide
概要
説明
ジアゾキシドは、主に高インスリン血症による低血糖の管理に使用される、非利尿性ベンゾチアジアジン誘導体です。 化学的にはチアジド系利尿剤と関連していますが、炭酸脱水酵素を阻害せず、塩化物利尿作用やナトリウム利尿作用もありません . ジアゾキシドは、ATP感受性カリウムチャネルを活性化することで、インスリンの放出を阻害することが知られています .
2. 製法
ジアゾキシドの製法には、いくつかの合成経路があります。一般的な方法の1つは、o-アミノベンゼンスルホンアミドとN-クロロスクシンイミドを塩素系溶媒中で反応させて、2-アミノ-5-クロロベンゼンスルホンアミドを得ることです。 この中間体をイミダゾール塩とアミド系溶媒と混合し、加熱してジアゾキシドを得ます . この方法では、高腐食性で有毒な試薬の使用を避け、工業生産に適しています .
3. 化学反応解析
ジアゾキシドは、以下を含むさまざまな化学反応を起こします。
酸化: ジアゾキシドは特定の条件下で酸化される可能性がありますが、詳細な経路はあまり議論されていません。
還元: ジアゾキシドを含む還元反応はあまり報告されていません。
置換: ジアゾキシドは、特に官能基を含む置換反応に関与する可能性があります。これらの反応で使用される一般的な試薬には、N-クロロスクシンイミドとイミダゾール塩があります
作用機序
ジアゾキシドは、膵臓のβ細胞にあるATP感受性カリウムチャネルを活性化することで作用します。この活性化によりカリウムチャネルが開き、細胞膜の過分極が起こります。 その結果、カルシウム流入が減少し、インスリンの放出が阻害されます . この機序は、インスリンの放出を増加させるスルホニル尿素とは逆です .
6. 類似化合物の比較
ジアゾキシドは化学的にチアジド系利尿剤に似ていますが、利尿作用がありません。類似の化合物には以下が含まれます。
チアジド系利尿剤: 高血圧や浮腫の治療に使用される、ヒドロクロロチアジドなどですが、作用機序が異なります。
スルホニル尿素: グリベンクラミドなど、インスリンの放出を増加させ、2型糖尿病の治療に使用されます. ジアゾキシドは、利尿作用がなく、インスリンの放出を阻害するユニークな能力を備えており、これらの類似化合物とは異なります.
生化学分析
Biochemical Properties
Diazoxide activates ATP-sensitive potassium channels . This activation leads to the hyperpolarization of cell membranes, which in turn inhibits the release of insulin . This biochemical property makes this compound particularly useful in the treatment of hyperinsulinemic hypoglycemia .
Cellular Effects
This compound has a significant impact on various types of cells and cellular processes. It influences cell function by inhibiting insulin release . This inhibition can affect cell signaling pathways, gene expression, and cellular metabolism . This compound also exhibits hypotensive activity and reduces arteriolar smooth muscle and vascular resistance .
Molecular Mechanism
This compound works by binding to the sulfonylurea receptor (SUR) subunit of the ATP-sensitive potassium channel (K ATP) channel on the membrane of pancreatic beta‐cells . This binding promotes a potassium efflux from beta-cells, leading to the inhibition of insulin release .
Temporal Effects in Laboratory Settings
The effects of this compound can change over time in laboratory settings. The plasma half-life of this compound varies from 9.5 to 24 hours in children with normal renal function and from 20 to 72 hours in adults with normal renal function . This indicates that the product’s stability and degradation, as well as any long-term effects on cellular function, need to be considered in both in vitro and in vivo studies .
Metabolic Pathways
This compound is metabolized in the liver through oxidation of the 3-methyl group, producing hydroxymethyl (MI) and carboxy (M2) derivatives . The MI derivatives undergo subsequent sulphate conjugation . It is estimated that, in subjects with normal renal function, 54-60% of this compound is metabolized .
Subcellular Localization
This compound is known to affect mitochondrial bioenergetics by opening the mKATP channel This suggests that this compound may be localized in the mitochondria of cells
準備方法
The preparation of diazoxide involves several synthetic routes. One common method includes reacting o-aminobenzenesulfonamide with N-chlorosuccinimide in a chlorine solvent to obtain 2-amino-5-chlorobenzenesulfonamide. This intermediate is then mixed with an imidazole salt and an amide solvent, followed by heating to obtain this compound . This method avoids the use of highly corrosive and toxic reagents, making it more suitable for industrial production .
化学反応の分析
Diazoxide undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions, although detailed pathways are less commonly discussed.
Reduction: Reduction reactions involving this compound are not widely reported.
Substitution: This compound can participate in substitution reactions, particularly involving its functional groups. Common reagents used in these reactions include N-chlorosuccinimide and imidazole salts
科学的研究の応用
ジアゾキシドは、科学研究で幅広く応用されています。
化学: さまざまな合成経路で使用され、多成分反応の試薬としても使用されます.
生物学: 細胞のカリウムチャネルに対する影響と、細胞活動の調節における役割について研究されています。
医学: 主にインスリンの放出を阻害することにより、高インスリン血症性低血糖の治療に使用されます.
産業: 医薬品の製造に使用され、創薬における研究ツールとしても使用されます。
類似化合物との比較
Diazoxide is chemically similar to thiazide diuretics but differs in its lack of diuretic activity. Similar compounds include:
Thiazide diuretics: Such as hydrochlorothiazide, which are used to treat hypertension and edema but have different mechanisms of action.
Sulfonylureas: Such as glibenclamide, which increase insulin release and are used to treat type 2 diabetes. This compound’s unique ability to inhibit insulin release while lacking diuretic activity sets it apart from these similar compounds.
特性
IUPAC Name |
7-chloro-3-methyl-4H-1λ6,2,4-benzothiadiazine 1,1-dioxide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H7ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-4H,1H3,(H,10,11) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GDLBFKVLRPITMI-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NS(=O)(=O)C2=C(N1)C=CC(=C2)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H7ClN2O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7022914 | |
| Record name | Diazoxide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022914 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
230.67 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
5.52e-01 g/L | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane. Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity. It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance. | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
364-98-7 | |
| Record name | Diazoxide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=364-98-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diazoxide [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000364987 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759574 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=76130 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=64198 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Diazoxide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022914 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diazoxide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.006.063 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DIAZOXIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/O5CB12L4FN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
330.5 °C | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary target of diazoxide, and how does this interaction affect pancreatic β-cells?
A1: this compound primarily targets mitochondrial ATP-sensitive potassium (mitoKATP) channels [, , , ]. By binding to and opening these channels, this compound induces hyperpolarization of the β-cell membrane, effectively inhibiting insulin secretion [, , , , ]. This mechanism is thought to involve increased potassium permeability, ultimately reducing calcium influx and blocking insulin release [, , , ].
Q2: Beyond mitoKATP channels, does this compound interact with other targets to influence cellular processes?
A2: Research suggests that this compound might directly interact with mitochondrial F0F1 ATP synthase, promoting the binding of the inhibitor protein IF(1) and reversibly inhibiting ATP hydrolysis []. This interaction could contribute to the cardioprotective effects observed with this compound treatment [, , ].
Q3: this compound is known to influence calcium signaling in various cell types. How does this occur?
A3: this compound can modulate calcium signaling through multiple pathways. In rat ventricular myocytes, this compound has been shown to modulate the opening of the mitochondrial permeability transition pore, leading to an increase in calcium transients independently of changes in mitochondrial membrane potential []. In endothelial cells, this compound can increase intracellular calcium levels through both mitochondrial reactive oxygen species-dependent and -independent mechanisms, leading to activation of endothelial nitric oxide synthase (eNOS) and subsequent vasodilation [].
Q4: What is the molecular formula and weight of this compound?
A4: this compound has the molecular formula C8H7ClN2O3S and a molecular weight of 230.66 g/mol.
Q5: How do structural modifications of this compound impact its activity and selectivity for KATP channels?
A5: While the provided papers don't directly compare different this compound analogs, they highlight the importance of specific structural features. For instance, the presence of the sulfonylurea moiety is crucial for its interaction with KATP channels []. Further research exploring SAR could reveal modifications that enhance selectivity for mitoKATP channels over other subtypes.
Q6: What is the primary route of administration for this compound, and what is known about its pharmacokinetic profile?
A7: this compound can be administered intravenously or orally [, ]. It exhibits rapid but transient effects on plasma insulin and glucose levels, suggesting a short half-life []. One study observed that this compound crosses the placental barrier and is present in amniotic fluid and urine during fetal exposure [].
Q7: What are the documented effects of this compound on blood glucose and insulin levels in animal models?
A8: this compound consistently induces hyperglycemia in various animal models, including rats and mice [, ]. This effect is attributed to its inhibitory action on insulin secretion from pancreatic β-cells [, , ]. Notably, the magnitude of hyperglycemia can be modulated by calcium receptor activity [].
Q8: What is the clinical significance of this compound responsiveness in patients with congenital hyperinsulinism (CHI)?
A9: this compound responsiveness is a crucial factor in the management of CHI [, , ]. Patients with KATP-hyperinsulinism, particularly those with mutations in the ABCC8 or KCNJ11 genes, often exhibit this compound unresponsiveness []. This characteristic can guide treatment decisions, as this compound may be less effective in these cases, necessitating alternative therapies like octreotide or surgery [, ].
Q9: Have any adverse effects been reported in association with this compound treatment?
A10: While the provided papers don't extensively discuss adverse effects, some studies mention alopecia and hypertrichosis lanuginosa as potential side effects observed in infants exposed to this compound in utero []. Additionally, one study reports a case of this compound-induced neutropenia after prolonged treatment []. These findings highlight the importance of monitoring for potential side effects during this compound therapy.
Q10: Are there known mechanisms of resistance to this compound, particularly in the context of CHI?
A11: this compound unresponsiveness is a significant challenge in CHI management. Mutations in the ABCC8 and KCNJ11 genes, encoding subunits of the KATP channel, are strongly associated with this compound resistance [, ]. This suggests that these mutations disrupt the drug's ability to bind to and activate the channel, rendering it ineffective in suppressing insulin secretion.
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![{2-Butyl-5-chloro-3-[2'-(1-trityl-1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-imidazol-4-yl}-methanol](/img/structure/B193138.png)
![Des[2'-(1H-tetrazol-5-yl)] 2-Cyanolosartan Carboxaldehyde](/img/structure/B193153.png)
![5-(4'-Methyl-[1,1'-biphenyl]-2-yl)-1-trityl-1H-tetrazole](/img/structure/B193154.png)