001, Everolimus
Übersicht
Beschreibung
Everolimus is a derivative of rapamycin, known for its immunosuppressive and anti-proliferative properties. It is primarily used to prevent organ rejection in transplant patients and to treat various types of cancer, including renal cell carcinoma and certain types of breast cancer . Everolimus works by inhibiting the mammalian target of rapamycin (mTOR), a key protein involved in cell growth and proliferation .
Wirkmechanismus
Target of Action
Everolimus, also known as RAD001, primarily targets the mammalian target of rapamycin (mTOR) . mTOR is a serine/threonine kinase that plays a crucial role in regulating cell growth, proliferation, and survival .
Mode of Action
Everolimus works by forming a complex with the FK506 binding protein-12 (FKBP-12), which inhibits the activation of mTOR . By inhibiting mTOR, Everolimus disrupts several cellular processes, including protein synthesis and cell cycle progression .
Biochemical Pathways
The mTOR pathway, which Everolimus inhibits, is involved in several key cellular processes . These include:
By inhibiting mTOR, Everolimus can disrupt these processes, leading to a decrease in cell growth and proliferation .
Pharmacokinetics
Everolimus exhibits significant inter-individual pharmacokinetic variability . It is lipophilic and rapidly absorbed into the arterial wall . It is also a substrate of both CYP3A4 and PgP, and inhibits the activity of CYP3A4 . This means that its metabolism can be affected by other drugs that interact with these enzymes .
Result of Action
The inhibition of mTOR by Everolimus leads to a decrease in cell growth and proliferation . This makes it effective in treating various types of malignancies, including renal cell carcinoma, neuroendocrine tumors, and hormone receptor-positive, HER2-negative breast cancer .
Action Environment
The action of Everolimus is influenced by environmental factors. The mTOR pathway, which Everolimus inhibits, regulates cell growth and metabolism in response to environmental factors . Furthermore, the pharmacokinetics of Everolimus can be affected by factors such as diet and the presence of other drugs .
Wissenschaftliche Forschungsanwendungen
Everolimus hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung für die Untersuchung von mTOR-Inhibitoren und deren chemischen Eigenschaften verwendet.
Biologie: Untersucht für seine Rolle in Zellwachstums-, Proliferations- und Überlebenswegen.
Medizin: Weit verbreitet im klinischen Umfeld zur Verhinderung von Organabstoßung und zur Behandlung von Krebsarten.
5. Wirkmechanismus
Everolimus übt seine Wirkungen aus, indem es den mTOR-Signalweg hemmt, insbesondere den mTORC1-Proteinkomplex. Diese Hemmung stört die Signale für Zellwachstum, -proliferation und -überleben, was zu einem reduzierten Tumorwachstum und einer Modulation der Immunantwort führt. Die molekularen Zielstrukturen umfassen verschiedene Proteine, die am PI3K/AKT/mTOR-Signalweg beteiligt sind .
Ähnliche Verbindungen:
Sirolimus (Rapamycin): Die Stammverbindung von Everolimus, die für ähnliche therapeutische Zwecke verwendet wird, aber unterschiedliche pharmakokinetische Eigenschaften aufweist.
Temsirolimus: Ein weiterer mTOR-Inhibitor mit einem ähnlichen Wirkmechanismus, der jedoch hauptsächlich zur Behandlung von Nierenzellkarzinomen eingesetzt wird.
Einzigartigkeit von Everolimus: Everolimus ist einzigartig aufgrund seiner verbesserten oralen Bioverfügbarkeit und der spezifischen Zielrichtung des mTORC1-Proteinkomplexes. Diese Spezifität ermöglicht eine effektivere Hemmung von Zellwachstum und -proliferation, was es zu einem wertvollen therapeutischen Mittel in der Onkologie und Transplantationsmedizin macht .
Biochemische Analyse
Biochemical Properties
Everolimus plays a crucial role in biochemical reactions by inhibiting the mTORC1 protein complex, which is involved in cell growth, proliferation, and survival. It interacts with several enzymes, proteins, and biomolecules, including FK506 binding protein 12 (FKBP12), mTORC1, and hypoxia-inducible factor 1-alpha (HIF-1α). The interaction between Everolimus and FKBP12 forms a complex that binds to mTORC1, inhibiting its activity. This inhibition leads to a decrease in protein synthesis, cell proliferation, and angiogenesis .
Cellular Effects
Everolimus affects various types of cells and cellular processes. It influences cell function by inhibiting the mTORC1 pathway, which plays a critical role in cell growth, proliferation, and metabolism. Everolimus reduces the phosphorylation of downstream targets such as p70S6 kinase and 4E-BP1, leading to decreased protein synthesis and cell cycle progression. It also affects gene expression by downregulating HIF-1α and vascular endothelial growth factor (VEGF), resulting in reduced angiogenesis .
Molecular Mechanism
The molecular mechanism of Everolimus involves its binding to FKBP12, forming a complex that inhibits mTORC1. This inhibition disrupts the mTOR signaling pathway, leading to reduced protein synthesis, cell growth, and proliferation. Everolimus also affects other cellular processes such as autophagy, lipid biosynthesis, and mitochondrial biogenesis. By inhibiting mTORC1, Everolimus reduces the activity of S6 kinase and 4E-BP1, which are essential for protein translation and cell cycle progression .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Everolimus change over time. Everolimus is stable under physiological conditions, but its degradation can occur in the presence of certain enzymes and environmental factors. Long-term studies have shown that Everolimus maintains its inhibitory effects on mTORC1, leading to sustained reductions in cell proliferation and angiogenesis. Prolonged exposure to Everolimus can result in adaptive resistance mechanisms, such as upregulation of alternative signaling pathways .
Dosage Effects in Animal Models
The effects of Everolimus vary with different dosages in animal models. At low doses, Everolimus effectively inhibits mTORC1 activity, leading to reduced cell proliferation and angiogenesis. At high doses, Everolimus can cause toxic effects, including immunosuppression, hyperlipidemia, and impaired wound healing. Studies in animal models have shown that Everolimus has a dose-dependent effect on tumor growth, with higher doses leading to greater inhibition of tumor progression .
Metabolic Pathways
Everolimus is involved in several metabolic pathways, including glycolysis, the pentose phosphate pathway, and the tricarboxylic acid cycle. It interacts with enzymes such as hexokinase, phosphofructokinase, and pyruvate kinase, leading to alterations in glucose metabolism. Everolimus impairs glucose utilization by reducing the activity of key glycolytic enzymes and decreasing the levels of intracellular glycometabolites .
Transport and Distribution
Everolimus is transported and distributed within cells and tissues through various mechanisms. It is a substrate of the cytochrome P450 enzyme CYP3A4 and the efflux transporter P-glycoprotein (PgP). Everolimus has a high volume of distribution and is extensively bound to plasma proteins. It is distributed to various tissues, including the liver, kidneys, and lungs. The transport and distribution of Everolimus are influenced by factors such as drug-drug interactions and genetic polymorphisms .
Subcellular Localization
Everolimus is localized in various subcellular compartments, including the cytoplasm, endoplasmic reticulum, and mitochondria. It affects the activity and function of these organelles by modulating the mTOR signaling pathway. Everolimus enhances the expression of proteins involved in microtubule stabilization, such as tubulin beta 2B class IIb (TUBB2B) and doublecortin domain containing 2 (DCDC2), which play a role in maintaining cytoskeletal integrity .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Everolimus is synthesized from rapamycin through a series of chemical reactions. The primary synthetic route involves the reaction of rapamycin with ethylene glycol mono trifluoromethanesulfonate under the influence of an organic base. This reaction produces an intermediate compound, which is then subjected to an acetal protection group removal reaction under acidic conditions to yield everolimus .
Industrial Production Methods: The industrial production of everolimus follows similar synthetic routes but is optimized for higher yields and cost-effectiveness. The process involves the use of inexpensive and readily available reagents, such as ethylene glycol mono trifluoromethanesulfonate, and includes steps for the recycling of unreacted rapamycin to ensure product quality and reduce costs .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Everolimus durchläuft verschiedene chemische Reaktionen, darunter Oxidation, Reduktion und Substitution. Diese Reaktionen sind für seine metabolische Verarbeitung und therapeutische Wirkung unerlässlich.
Häufige Reagenzien und Bedingungen:
Oxidation: Umfasst typischerweise die Verwendung von Oxidationsmitteln wie Wasserstoffperoxid oder Kaliumpermanganat.
Reduktion: Oft mit Reduktionsmitteln wie Natriumborhydrid oder Lithiumaluminiumhydrid durchgeführt.
Substitution: Beinhaltet nukleophile oder elektrophile Reagenzien unter kontrollierten Bedingungen.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene Metaboliten, die im Körper weiterverarbeitet werden, um ihre therapeutischen Wirkungen auszuüben .
Vergleich Mit ähnlichen Verbindungen
Sirolimus (Rapamycin): The parent compound of everolimus, used for similar therapeutic purposes but with different pharmacokinetic properties.
Temsirolimus: Another mTOR inhibitor with a similar mechanism of action but used primarily for treating renal cell carcinoma.
Uniqueness of Everolimus: Everolimus is unique due to its enhanced oral bioavailability and specific targeting of the mTORC1 protein complex. This specificity allows for more effective inhibition of cell growth and proliferation, making it a valuable therapeutic agent in oncology and transplant medicine .
Eigenschaften
CAS-Nummer |
159351-69-6 |
|---|---|
Molekularformel |
C53H83NO14 |
Molekulargewicht |
958.2 g/mol |
IUPAC-Name |
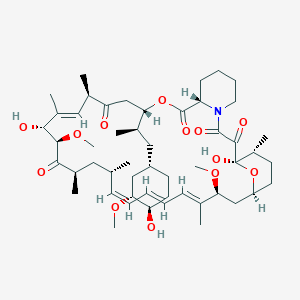
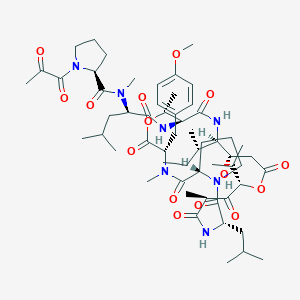
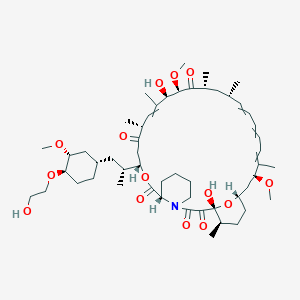
(1R)-1,18-dihydroxy-12-[1-[4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone |
InChI |
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/t32?,34?,35?,36?,38?,39?,40?,41?,43?,44?,45?,46?,48?,49?,53-/m1/s1 |
InChI-Schlüssel |
HKVAMNSJSFKALM-VUDSBINYSA-N |
SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Isomerische SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)[C@@]1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Kanonische SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Aussehen |
White to off-white solid powder |
| Everolimus is currently used as an immunosuppressant to prevent rejection of organ transplants. In a similar fashion to other mTOR inhibitors Everolimus' effect is solely on the mTORC1 protein and not on the mTORC2 protein. | |
Siedepunkt |
998.7±75.0 °C at 760 mmHg |
melting_point |
N/A |
| 159351-69-6 | |
Piktogramme |
Health Hazard |
Reinheit |
>98% (or refer to the Certificate of Analysis) |
Haltbarkeit |
Stable under recommended storage conditions. |
Löslichkeit |
Soluble in DMSO, not in water |
Lagerung |
−20°C |
Synonyme |
001, RAD 40-O-(2-hydroxyethyl)-rapamycin Afinitor Certican everolimus RAD 001 RAD, SDZ RAD001 SDZ RAD SDZ-RAD |
Herkunft des Produkts |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the mechanism of action of everolimus?
A: Everolimus is a potent inhibitor of the mammalian target of rapamycin (mTOR), specifically mTOR complex 1 (mTORC1) [, , ]. It acts by binding to the FKBP12 protein, forming a complex that inhibits the kinase activity of mTORC1.
Q2: What are the downstream effects of everolimus-mediated mTORC1 inhibition?
A2: Inhibition of mTORC1 by everolimus leads to a cascade of downstream effects, including:
- Reduced cell proliferation: Everolimus inhibits the phosphorylation of ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), key regulators of protein synthesis and cell cycle progression [, ]. This ultimately leads to reduced cell proliferation in various cell types, including tumor cells [, ].
- Inhibition of angiogenesis: Everolimus downregulates the expression of vascular endothelial growth factor (VEGF), a crucial pro-angiogenic factor, thereby suppressing angiogenesis [, ].
- Modulation of immune response: While not a primary focus in the provided articles, mTORC1 inhibition by everolimus has been shown to influence T cell differentiation and function [].
Q3: What is the molecular formula and weight of everolimus?
A3: The molecular formula of everolimus is C53H83NO14, and its molecular weight is 958.2 g/mol.
Q4: Is there any information available about spectroscopic data for everolimus in these research papers?
A4: The provided research papers primarily focus on the clinical and biological aspects of everolimus and do not delve into detailed spectroscopic characterization.
Q5: What is known about the stability of everolimus?
A: Everolimus exhibits stability challenges, requiring careful consideration during formulation and storage [, , ].
Q6: What strategies can enhance the stability, solubility, or bioavailability of everolimus?
A6: Several formulation approaches are employed to improve everolimus's pharmaceutical properties:
- Solid dispersions: By dispersing everolimus within a matrix of inert carriers, its dissolution rate and solubility can be enhanced [].
- Nanoparticulate systems: Encapsulating everolimus in nanoparticles offers advantages such as improved solubility, controlled release, and potential for targeted delivery [, ].
- Cyclodextrin complexation: Forming complexes with cyclodextrins can increase everolimus's aqueous solubility, enhancing its bioavailability [].
Q7: How is everolimus absorbed, distributed, metabolized, and excreted (ADME)?
A: Everolimus is an orally administered drug that demonstrates good absorption but substantial interindividual variability in its pharmacokinetics [, , , ].
- Absorption: Everolimus is well absorbed after oral administration, reaching peak concentrations in approximately 1-2 hours []. Food intake can influence its absorption [].
- Distribution: It exhibits a large volume of distribution, indicating extensive tissue distribution [].
- Metabolism: Everolimus is primarily metabolized in the liver by the cytochrome P450 (CYP) 3A4 enzyme [, , ].
- Excretion: It is excreted predominantly in feces, with a small fraction eliminated in urine [].
Q8: How is the therapeutic drug monitoring of everolimus conducted?
A: Therapeutic drug monitoring (TDM) of everolimus is routinely performed using validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods to measure drug levels in whole blood samples [, , ]. This information helps personalize dosage, manage drug interactions, and minimize toxicity.
Q9: Are there known drug interactions with everolimus?
A: Everolimus is a substrate of CYP3A4, and co-administration with CYP3A4 inhibitors or inducers can alter its blood levels [, , , ]. Dosage adjustments might be necessary when used concomitantly with these agents.
Q10: What in vitro and in vivo models have been used to study everolimus's anticancer activity?
A10: Everolimus's anticancer activity has been investigated in various in vitro and in vivo models, including:
- Cell-based assays: Researchers have employed cell proliferation assays, colony formation assays, and spheroid growth assays to assess everolimus's inhibitory effects on tumor cell growth [, , ].
- Animal models: Everolimus has demonstrated efficacy in preclinical animal models of various cancers, including renal cell carcinoma, pancreatic neuroendocrine tumors, and breast cancer [, , , ]. These models provide valuable insights into the drug's pharmacodynamic effects and potential for therapeutic benefit.
Q11: Are there clinical trials demonstrating the efficacy of everolimus in cancer treatment?
A11: Yes, several randomized controlled trials have established the efficacy of everolimus in specific cancer types:
- Renal cell carcinoma (RCC): Everolimus has shown significant clinical benefit in patients with advanced RCC who progressed on prior VEGF-targeted therapy, leading to its approval for this indication [, , , , ].
- Pancreatic neuroendocrine tumors (pNETs): Everolimus has demonstrated significant improvement in progression-free survival in patients with advanced pNETs, leading to its approval for this indication [, , ].
- Breast cancer: Everolimus, in combination with exemestane, has shown efficacy in postmenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer resistant to aromatase inhibitor therapy [, , ].
Q12: What are the known mechanisms of resistance to everolimus?
A12: Resistance to everolimus can develop through various mechanisms, including:
- Activation of alternative signaling pathways: Tumor cells may circumvent mTORC1 inhibition by activating alternative pro-survival and proliferative pathways, such as the PI3K/Akt pathway [, ].
- Mutations in mTOR pathway components: Mutations in genes encoding components of the mTOR pathway, such as mTOR itself or its downstream effectors, can contribute to resistance [, ].
Q13: Are there biomarkers to predict everolimus efficacy or monitor treatment response?
A13: Research is ongoing to identify reliable biomarkers for everolimus:
- FDG-PET SUV changes: Early changes in standardized uptake value (SUV) on 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scans might hold promise as a predictive biomarker for long-term benefit in patients with hormone receptor-positive metastatic breast cancer treated with everolimus and exemestane [].
Q14: What are the alternatives and substitutes for everolimus?
A: Sirolimus (rapamycin), another mTOR inhibitor, shares a similar mechanism of action and has shown comparable efficacy in some clinical settings [, , ]. The choice between everolimus and sirolimus often depends on patient-specific factors, treatment history, and the specific clinical scenario.
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![(2S,3'R,3aS,4S,4'S,5'R,6R,6'R,7S,7aS)-4-[(1R,2S,3R,5S,6R)-3-amino-2,6-dihydroxy-5-(methylamino)cyclohexyl]oxy-6'-[(1R)-1-amino-2-hydroxyethyl]-6-(hydroxymethyl)spiro[4,6,7,7a-tetrahydro-3aH-[1,3]dioxolo[4,5-c]pyran-2,2'-oxane]-3',4',5',7-tetrol](/img/structure/B549156.png)
![N-[(3S,6S,9S,11R,15S,18S,20R,21R,24S,25S)-3-[(1R)-3-amino-1-hydroxy-3-oxopropyl]-6-[(1S,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl]-11,20,21,25-tetrahydroxy-15-[(1R)-1-hydroxyethyl]-2,5,8,14,17,23-hexaoxo-1,4,7,13,16,22-hexazatricyclo[22.3.0.09,13]heptacosan-18-yl]-10,12-dimethyltetradecanamide](/img/structure/B549162.png)
![N-[(3S,9S,11R,18S,20R,21R,24S,25S)-21-(2-Aminoethylamino)-3-[(1R)-3-amino-1-hydroxypropyl]-6-[(1S,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl]-11,20,25-trihydroxy-15-[(1R)-1-hydroxyethyl]-2,5,8,14,17,23-hexaoxo-1,4,7,13,16,22-hexazatricyclo[22.3.0.09,13]heptacosan-18-yl]-10,12-dimethyltetradecanamide](/img/structure/B549164.png)