001, RAD
Descripción general
Descripción
Everolimus es un derivado de la rapamicina, conocido por sus propiedades inmunosupresoras y antiproliferativas. Se utiliza principalmente para prevenir el rechazo de órganos en pacientes trasplantados y para tratar varios tipos de cáncer, incluyendo el carcinoma de células renales y ciertos tipos de cáncer de mama . Everolimus funciona inhibiendo la diana mamífera de la rapamicina (mTOR), una proteína clave involucrada en el crecimiento y proliferación celular .
Mecanismo De Acción
Everolimus ejerce sus efectos inhibiendo la vía mTOR, específicamente dirigiéndose al complejo proteico mTORC1. Esta inhibición interrumpe las señales de crecimiento, proliferación y supervivencia celular, lo que lleva a una reducción del crecimiento tumoral y a la modulación de la respuesta inmunitaria. Los objetivos moleculares incluyen varias proteínas involucradas en la vía de señalización PI3K/AKT/mTOR .
Compuestos Similares:
Sirolimus (Rapamicina): El compuesto padre de everolimus, utilizado para fines terapéuticos similares, pero con diferentes propiedades farmacocinéticas.
Temsirolimus: Otro inhibidor de mTOR con un mecanismo de acción similar, pero utilizado principalmente para tratar el carcinoma de células renales.
Singularidad de Everolimus: Everolimus es único debido a su biodisponibilidad oral mejorada y su orientación específica al complejo proteico mTORC1. Esta especificidad permite una inhibición más efectiva del crecimiento y proliferación celular, lo que lo convierte en un valioso agente terapéutico en oncología y medicina de trasplantes .
Aplicaciones Científicas De Investigación
Everolimus tiene una amplia gama de aplicaciones de investigación científica:
Química: Se utiliza como un compuesto modelo para estudiar los inhibidores de mTOR y sus propiedades químicas.
Biología: Se investiga por su papel en las vías de crecimiento, proliferación y supervivencia celular.
Medicina: Ampliamente utilizado en entornos clínicos para prevenir el rechazo de órganos y tratar cánceres.
Análisis Bioquímico
Biochemical Properties
Everolimus plays a crucial role in biochemical reactions by inhibiting the mTORC1 protein complex, which is involved in cell growth, proliferation, and survival. It interacts with several enzymes, proteins, and biomolecules, including FK506 binding protein 12 (FKBP12), mTORC1, and hypoxia-inducible factor 1-alpha (HIF-1α). The interaction between Everolimus and FKBP12 forms a complex that binds to mTORC1, inhibiting its activity. This inhibition leads to a decrease in protein synthesis, cell proliferation, and angiogenesis .
Cellular Effects
Everolimus affects various types of cells and cellular processes. It influences cell function by inhibiting the mTORC1 pathway, which plays a critical role in cell growth, proliferation, and metabolism. Everolimus reduces the phosphorylation of downstream targets such as p70S6 kinase and 4E-BP1, leading to decreased protein synthesis and cell cycle progression. It also affects gene expression by downregulating HIF-1α and vascular endothelial growth factor (VEGF), resulting in reduced angiogenesis .
Molecular Mechanism
The molecular mechanism of Everolimus involves its binding to FKBP12, forming a complex that inhibits mTORC1. This inhibition disrupts the mTOR signaling pathway, leading to reduced protein synthesis, cell growth, and proliferation. Everolimus also affects other cellular processes such as autophagy, lipid biosynthesis, and mitochondrial biogenesis. By inhibiting mTORC1, Everolimus reduces the activity of S6 kinase and 4E-BP1, which are essential for protein translation and cell cycle progression .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Everolimus change over time. Everolimus is stable under physiological conditions, but its degradation can occur in the presence of certain enzymes and environmental factors. Long-term studies have shown that Everolimus maintains its inhibitory effects on mTORC1, leading to sustained reductions in cell proliferation and angiogenesis. Prolonged exposure to Everolimus can result in adaptive resistance mechanisms, such as upregulation of alternative signaling pathways .
Dosage Effects in Animal Models
The effects of Everolimus vary with different dosages in animal models. At low doses, Everolimus effectively inhibits mTORC1 activity, leading to reduced cell proliferation and angiogenesis. At high doses, Everolimus can cause toxic effects, including immunosuppression, hyperlipidemia, and impaired wound healing. Studies in animal models have shown that Everolimus has a dose-dependent effect on tumor growth, with higher doses leading to greater inhibition of tumor progression .
Metabolic Pathways
Everolimus is involved in several metabolic pathways, including glycolysis, the pentose phosphate pathway, and the tricarboxylic acid cycle. It interacts with enzymes such as hexokinase, phosphofructokinase, and pyruvate kinase, leading to alterations in glucose metabolism. Everolimus impairs glucose utilization by reducing the activity of key glycolytic enzymes and decreasing the levels of intracellular glycometabolites .
Transport and Distribution
Everolimus is transported and distributed within cells and tissues through various mechanisms. It is a substrate of the cytochrome P450 enzyme CYP3A4 and the efflux transporter P-glycoprotein (PgP). Everolimus has a high volume of distribution and is extensively bound to plasma proteins. It is distributed to various tissues, including the liver, kidneys, and lungs. The transport and distribution of Everolimus are influenced by factors such as drug-drug interactions and genetic polymorphisms .
Subcellular Localization
Everolimus is localized in various subcellular compartments, including the cytoplasm, endoplasmic reticulum, and mitochondria. It affects the activity and function of these organelles by modulating the mTOR signaling pathway. Everolimus enhances the expression of proteins involved in microtubule stabilization, such as tubulin beta 2B class IIb (TUBB2B) and doublecortin domain containing 2 (DCDC2), which play a role in maintaining cytoskeletal integrity .
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción: Everolimus se sintetiza a partir de la rapamicina mediante una serie de reacciones químicas. La ruta sintética principal implica la reacción de la rapamicina con mono trifluorometanosulfonato de etilenglicol bajo la influencia de una base orgánica. Esta reacción produce un compuesto intermedio, que luego se somete a una reacción de eliminación del grupo protector acetal en condiciones ácidas para producir everolimus .
Métodos de Producción Industrial: La producción industrial de everolimus sigue rutas sintéticas similares, pero está optimizada para obtener mayores rendimientos y rentabilidad. El proceso involucra el uso de reactivos económicos y fácilmente disponibles, como el mono trifluorometanosulfonato de etilenglicol, e incluye pasos para el reciclaje de la rapamicina no reaccionada para garantizar la calidad del producto y reducir los costos .
Análisis De Reacciones Químicas
Tipos de Reacciones: Everolimus experimenta varias reacciones químicas, incluyendo oxidación, reducción y sustitución. Estas reacciones son esenciales para su procesamiento metabólico y acción terapéutica.
Reactivos y Condiciones Comunes:
Oxidación: Generalmente involucra el uso de agentes oxidantes como el peróxido de hidrógeno o el permanganato de potasio.
Reducción: A menudo se lleva a cabo utilizando agentes reductores como el borohidruro de sodio o el hidruro de litio y aluminio.
Sustitución: Implica reactivos nucleófilos o electrófilos en condiciones controladas.
Productos Principales: Los productos primarios formados a partir de estas reacciones incluyen varios metabolitos que se procesan aún más en el cuerpo para ejercer sus efectos terapéuticos .
Comparación Con Compuestos Similares
Sirolimus (Rapamycin): The parent compound of everolimus, used for similar therapeutic purposes but with different pharmacokinetic properties.
Temsirolimus: Another mTOR inhibitor with a similar mechanism of action but used primarily for treating renal cell carcinoma.
Uniqueness of Everolimus: Everolimus is unique due to its enhanced oral bioavailability and specific targeting of the mTORC1 protein complex. This specificity allows for more effective inhibition of cell growth and proliferation, making it a valuable therapeutic agent in oncology and transplant medicine .
Propiedades
Número CAS |
159351-69-6 |
|---|---|
Fórmula molecular |
C53H83NO14 |
Peso molecular |
958.2 g/mol |
Nombre IUPAC |
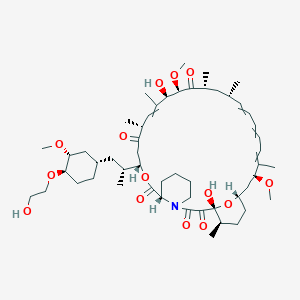
(1R)-1,18-dihydroxy-12-[1-[4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone |
InChI |
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/t32?,34?,35?,36?,38?,39?,40?,41?,43?,44?,45?,46?,48?,49?,53-/m1/s1 |
Clave InChI |
HKVAMNSJSFKALM-VUDSBINYSA-N |
SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
SMILES isomérico |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)[C@@]1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
SMILES canónico |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Apariencia |
White to off-white solid powder |
| Everolimus is currently used as an immunosuppressant to prevent rejection of organ transplants. In a similar fashion to other mTOR inhibitors Everolimus' effect is solely on the mTORC1 protein and not on the mTORC2 protein. | |
Punto de ebullición |
998.7±75.0 °C at 760 mmHg |
melting_point |
N/A |
| 159351-69-6 | |
Pictogramas |
Health Hazard |
Pureza |
>98% (or refer to the Certificate of Analysis) |
Vida útil |
Stable under recommended storage conditions. |
Solubilidad |
Soluble in DMSO, not in water |
Almacenamiento |
−20°C |
Sinónimos |
001, RAD 40-O-(2-hydroxyethyl)-rapamycin Afinitor Certican everolimus RAD 001 RAD, SDZ RAD001 SDZ RAD SDZ-RAD |
Origen del producto |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the mechanism of action of everolimus?
A: Everolimus is a potent inhibitor of the mammalian target of rapamycin (mTOR), specifically mTOR complex 1 (mTORC1) [, , ]. It acts by binding to the FKBP12 protein, forming a complex that inhibits the kinase activity of mTORC1.
Q2: What are the downstream effects of everolimus-mediated mTORC1 inhibition?
A2: Inhibition of mTORC1 by everolimus leads to a cascade of downstream effects, including:
- Reduced cell proliferation: Everolimus inhibits the phosphorylation of ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), key regulators of protein synthesis and cell cycle progression [, ]. This ultimately leads to reduced cell proliferation in various cell types, including tumor cells [, ].
- Inhibition of angiogenesis: Everolimus downregulates the expression of vascular endothelial growth factor (VEGF), a crucial pro-angiogenic factor, thereby suppressing angiogenesis [, ].
- Modulation of immune response: While not a primary focus in the provided articles, mTORC1 inhibition by everolimus has been shown to influence T cell differentiation and function [].
Q3: What is the molecular formula and weight of everolimus?
A3: The molecular formula of everolimus is C53H83NO14, and its molecular weight is 958.2 g/mol.
Q4: Is there any information available about spectroscopic data for everolimus in these research papers?
A4: The provided research papers primarily focus on the clinical and biological aspects of everolimus and do not delve into detailed spectroscopic characterization.
Q5: What is known about the stability of everolimus?
A: Everolimus exhibits stability challenges, requiring careful consideration during formulation and storage [, , ].
Q6: What strategies can enhance the stability, solubility, or bioavailability of everolimus?
A6: Several formulation approaches are employed to improve everolimus's pharmaceutical properties:
- Solid dispersions: By dispersing everolimus within a matrix of inert carriers, its dissolution rate and solubility can be enhanced [].
- Nanoparticulate systems: Encapsulating everolimus in nanoparticles offers advantages such as improved solubility, controlled release, and potential for targeted delivery [, ].
- Cyclodextrin complexation: Forming complexes with cyclodextrins can increase everolimus's aqueous solubility, enhancing its bioavailability [].
Q7: How is everolimus absorbed, distributed, metabolized, and excreted (ADME)?
A: Everolimus is an orally administered drug that demonstrates good absorption but substantial interindividual variability in its pharmacokinetics [, , , ].
- Absorption: Everolimus is well absorbed after oral administration, reaching peak concentrations in approximately 1-2 hours []. Food intake can influence its absorption [].
- Distribution: It exhibits a large volume of distribution, indicating extensive tissue distribution [].
- Metabolism: Everolimus is primarily metabolized in the liver by the cytochrome P450 (CYP) 3A4 enzyme [, , ].
- Excretion: It is excreted predominantly in feces, with a small fraction eliminated in urine [].
Q8: How is the therapeutic drug monitoring of everolimus conducted?
A: Therapeutic drug monitoring (TDM) of everolimus is routinely performed using validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods to measure drug levels in whole blood samples [, , ]. This information helps personalize dosage, manage drug interactions, and minimize toxicity.
Q9: Are there known drug interactions with everolimus?
A: Everolimus is a substrate of CYP3A4, and co-administration with CYP3A4 inhibitors or inducers can alter its blood levels [, , , ]. Dosage adjustments might be necessary when used concomitantly with these agents.
Q10: What in vitro and in vivo models have been used to study everolimus's anticancer activity?
A10: Everolimus's anticancer activity has been investigated in various in vitro and in vivo models, including:
- Cell-based assays: Researchers have employed cell proliferation assays, colony formation assays, and spheroid growth assays to assess everolimus's inhibitory effects on tumor cell growth [, , ].
- Animal models: Everolimus has demonstrated efficacy in preclinical animal models of various cancers, including renal cell carcinoma, pancreatic neuroendocrine tumors, and breast cancer [, , , ]. These models provide valuable insights into the drug's pharmacodynamic effects and potential for therapeutic benefit.
Q11: Are there clinical trials demonstrating the efficacy of everolimus in cancer treatment?
A11: Yes, several randomized controlled trials have established the efficacy of everolimus in specific cancer types:
- Renal cell carcinoma (RCC): Everolimus has shown significant clinical benefit in patients with advanced RCC who progressed on prior VEGF-targeted therapy, leading to its approval for this indication [, , , , ].
- Pancreatic neuroendocrine tumors (pNETs): Everolimus has demonstrated significant improvement in progression-free survival in patients with advanced pNETs, leading to its approval for this indication [, , ].
- Breast cancer: Everolimus, in combination with exemestane, has shown efficacy in postmenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer resistant to aromatase inhibitor therapy [, , ].
Q12: What are the known mechanisms of resistance to everolimus?
A12: Resistance to everolimus can develop through various mechanisms, including:
- Activation of alternative signaling pathways: Tumor cells may circumvent mTORC1 inhibition by activating alternative pro-survival and proliferative pathways, such as the PI3K/Akt pathway [, ].
- Mutations in mTOR pathway components: Mutations in genes encoding components of the mTOR pathway, such as mTOR itself or its downstream effectors, can contribute to resistance [, ].
Q13: Are there biomarkers to predict everolimus efficacy or monitor treatment response?
A13: Research is ongoing to identify reliable biomarkers for everolimus:
- FDG-PET SUV changes: Early changes in standardized uptake value (SUV) on 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scans might hold promise as a predictive biomarker for long-term benefit in patients with hormone receptor-positive metastatic breast cancer treated with everolimus and exemestane [].
Q14: What are the alternatives and substitutes for everolimus?
A: Sirolimus (rapamycin), another mTOR inhibitor, shares a similar mechanism of action and has shown comparable efficacy in some clinical settings [, , ]. The choice between everolimus and sirolimus often depends on patient-specific factors, treatment history, and the specific clinical scenario.
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![(2S,3'R,3aS,4S,4'S,5'R,6R,6'R,7S,7aS)-4-[(1R,2S,3R,5S,6R)-3-amino-2,6-dihydroxy-5-(methylamino)cyclohexyl]oxy-6'-[(1R)-1-amino-2-hydroxyethyl]-6-(hydroxymethyl)spiro[4,6,7,7a-tetrahydro-3aH-[1,3]dioxolo[4,5-c]pyran-2,2'-oxane]-3',4',5',7-tetrol](/img/structure/B549156.png)
![Pneumocandin A0, 1-[(4R,5R)-4,5-dihydroxy-N2-(1-oxohexadecyl)-L-ornithine]-4-[(4S)-4-hydroxy-4-[4-hydroxy-3-(sulfooxy)phenyl]-L-threonine]-](/img/structure/B549160.png)
![N-[(3S,9S,11R,18S,20R,21R,24S,25S)-21-(2-Aminoethylamino)-3-[(1R)-3-amino-1-hydroxypropyl]-6-[(1S,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl]-11,20,25-trihydroxy-15-[(1R)-1-hydroxyethyl]-2,5,8,14,17,23-hexaoxo-1,4,7,13,16,22-hexazatricyclo[22.3.0.09,13]heptacosan-18-yl]-10,12-dimethyltetradecanamide](/img/structure/B549164.png)