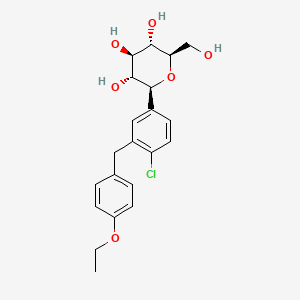
Dapagliflozina
Descripción general
Descripción
La dapagliflozina es un medicamento que se utiliza principalmente para tratar la diabetes mellitus tipo 2. Pertenece a la clase de inhibidores del cotransportador 2 de sodio-glucosa. Al inhibir este transportador, la this compound reduce la reabsorción de glucosa en los riñones, lo que lleva a una mayor excreción de glucosa en la orina. Esto ayuda a disminuir los niveles de glucosa en sangre. Además, la this compound se utiliza para tratar la insuficiencia cardíaca y la enfermedad renal crónica .
Aplicaciones Científicas De Investigación
La dapagliflozina tiene una amplia gama de aplicaciones de investigación científica:
Química: Se utiliza como compuesto modelo para estudiar el comportamiento de los inhibidores del cotransportador 2 de sodio-glucosa.
Biología: Se ha investigado por sus efectos sobre el metabolismo de la glucosa y la función renal.
Medicina: Se ha estudiado ampliamente por sus efectos terapéuticos en el tratamiento de la diabetes tipo 2, la insuficiencia cardíaca y la enfermedad renal crónica. .
Mecanismo De Acción
La dapagliflozina actúa inhibiendo el cotransportador 2 de sodio-glucosa en los túbulos renales proximales. Este transportador es responsable de la reabsorción de glucosa desde la luz tubular. Al inhibir este transportador, la this compound reduce la reabsorción de glucosa, lo que lleva a una mayor excreción de glucosa urinaria. Esto da como resultado niveles más bajos de glucosa en sangre. Además, la this compound reduce la reabsorción de sodio, lo que puede influir en varias funciones fisiológicas, incluida la reducción de la precarga y la poscarga del corazón y la regulación a la baja de la actividad simpática .
Compuestos similares:
- Empagliflozina
- Canagliflozina
- Ipragliflozina
Comparación:
- Empagliflozina: Tanto la this compound como la empagliflozina son inhibidores del cotransportador 2 de sodio-glucosa que se utilizan para tratar la diabetes tipo 2. La this compound ha mostrado un mayor riesgo de hospitalización por insuficiencia cardíaca en comparación con la empagliflozina .
- Canagliflozina: Al igual que la this compound, la canagliflozina se utiliza para tratar la diabetes tipo 2 y tiene beneficios adicionales para reducir los eventos cardiovasculares. La canagliflozina se ha asociado con un mayor riesgo de amputaciones de miembros inferiores.
- Ipragliflozina: Otro inhibidor del cotransportador 2 de sodio-glucosa con efectos similares de reducción de la glucosa. La this compound tiene un perfil de seguridad más favorable en términos de infecciones genitales .
La this compound destaca por sus beneficios integrales en el tratamiento de la diabetes tipo 2, la insuficiencia cardíaca y la enfermedad renal crónica, junto con su perfil de seguridad relativamente favorable.
Análisis Bioquímico
Biochemical Properties
Dapagliflozin plays a crucial role in biochemical reactions by inhibiting the SGLT2 protein, which is predominantly expressed in the proximal renal tubules. This inhibition prevents glucose reabsorption, leading to its excretion through urine. Dapagliflozin interacts with various enzymes and proteins, including sex hormone-binding globulin, transferrin receptor protein 1, disintegrin, metalloprotease-like decysin-1, and apolipoprotein A-IV, increasing their levels. Conversely, it decreases the levels of complement C3, fibronectin, afamin, attractin, xanthine, and uric acid .
Cellular Effects
Dapagliflozin influences various cell types and cellular processes. It enhances insulin sensitivity in hepatic and adipose tissues, leading to improved glucose metabolism. Dapagliflozin also affects cell signaling pathways, such as the MYD88, NLRP3 complex, Leukotrienes/Interleukin 6 axis, and mTORC1/Fox01/3a mediated apoptosis . These interactions result in changes in gene expression and cellular metabolism, contributing to its therapeutic effects.
Molecular Mechanism
At the molecular level, dapagliflozin exerts its effects by binding to the SGLT2 protein, inhibiting its function, and preventing glucose reabsorption in the kidneys. This inhibition leads to increased urinary glucose excretion, mimicking caloric restriction and promoting fat oxidation . Dapagliflozin also influences gene expression by modulating the activity of various transcription factors and signaling pathways, contributing to its beneficial effects on glucose metabolism and insulin sensitivity.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of dapagliflozin change over time. Studies have shown that dapagliflozin treatment for 12 weeks significantly decreases HbA1c, BMI, and HOMA-IR in patients with type 2 diabetes . The stability and degradation of dapagliflozin in vitro and in vivo settings are crucial for understanding its long-term effects on cellular function. Dapagliflozin has been shown to maintain its efficacy over extended periods, with consistent improvements in metabolic parameters.
Dosage Effects in Animal Models
The effects of dapagliflozin vary with different dosages in animal models. At therapeutic doses, dapagliflozin effectively reduces glucose levels and improves insulin sensitivity. At higher doses, it may cause adverse effects, such as dehydration and electrolyte imbalances. Studies have identified threshold effects, where the benefits of dapagliflozin plateau beyond a certain dosage, emphasizing the importance of optimal dosing for therapeutic efficacy .
Metabolic Pathways
Dapagliflozin is involved in several metabolic pathways, including glycolysis, gluconeogenesis, and the pentose phosphate pathway. It interacts with enzymes such as UGT1A9, which metabolizes dapagliflozin to its major inactive metabolite 3-O-glucuronide . These interactions influence metabolic flux and metabolite levels, contributing to the overall metabolic effects of dapagliflozin.
Transport and Distribution
Dapagliflozin is transported and distributed within cells and tissues through various transporters and binding proteins. It is primarily localized in the kidneys, where it exerts its glucose-lowering effects by inhibiting SGLT2. The distribution of dapagliflozin within other tissues, such as the liver and adipose tissue, also contributes to its metabolic effects .
Subcellular Localization
The subcellular localization of dapagliflozin is primarily in the proximal renal tubules, where it inhibits SGLT2. This localization is crucial for its activity and function, as it directly affects glucose reabsorption in the kidneys. Dapagliflozin may also undergo post-translational modifications that influence its targeting to specific compartments or organelles, further modulating its therapeutic effects .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La síntesis de la dapagliflozina implica varios pasos clave:
Preparación del reactivo de Grignard: La materia prima inicial, 1-cloro-2-(4-etoxi-bencil)-4-yodobenceno, reacciona con magnesio utilizando gránulos de yodo como iniciador para preparar un reactivo de Grignard.
Reacción de bromación: El azúcar peracetilado reacciona con bromuro de hidrógeno en una solución de ácido acético para preparar 2,3,4,6-tetraacetilglucosamina bromuro.
Formación del compuesto intermedio: El reactivo de Grignard se añade a una solución de metilbenceno/tetrahidrofurano, seguido de la adición de un catalizador de tierras raras y 2,3,4,6-tetraacetilglucosamina bromuro, dando como resultado un compuesto intermedio.
Hidrólisis y recristalización: El compuesto intermedio se somete a hidrólisis alcalina en una solución de hidróxido de sodio de tetrahidrofurano/etanol/agua, seguido de recristalización para obtener this compound.
Métodos de producción industrial: La producción industrial de this compound sigue rutas sintéticas similares pero a mayor escala, asegurando una alta pureza y rendimiento. El proceso implica estrictas medidas de control de calidad para minimizar las impurezas y garantizar la consistencia del producto final .
Tipos de reacciones:
Oxidación: La this compound puede sufrir reacciones de oxidación, lo que lleva a la formación de metabolitos como la oxo-dapagliflozina.
Reducción: Las reacciones de reducción pueden convertir la this compound en sus formas reducidas.
Sustitución: Las reacciones de sustitución pueden ocurrir en varias posiciones de la molécula de this compound, lo que lleva a la formación de diferentes derivados.
Reactivos y condiciones comunes:
Oxidación: Los agentes oxidantes comunes incluyen el peróxido de hidrógeno y el permanganato de potasio.
Reducción: Se utilizan agentes reductores como el borohidruro de sodio y el hidruro de litio y aluminio.
Sustitución: Se emplean diversos agentes halogenantes y nucleófilos en las reacciones de sustitución.
Productos principales:
This compound hidroxibencílica: Formada mediante hidroxilación.
Oxo-dapagliflozina: Formada mediante oxidación.
Desetil-dapagliflozina: Formada mediante desetilación.
Comparación Con Compuestos Similares
- Empagliflozin
- Canagliflozin
- Ipragliflozin
Comparison:
- Empagliflozin: Both dapagliflozin and empagliflozin are sodium-glucose co-transporter 2 inhibitors used to treat type 2 diabetes. dapagliflozin has shown a higher risk of hospitalization for heart failure compared to empagliflozin .
- Canagliflozin: Similar to dapagliflozin, canagliflozin is used to treat type 2 diabetes and has additional benefits in reducing cardiovascular events. canagliflozin has been associated with a higher risk of lower limb amputations.
- Ipragliflozin: Another sodium-glucose co-transporter 2 inhibitor with similar glucose-lowering effects. dapagliflozin has a more favorable safety profile in terms of genital infections .
Dapagliflozin stands out due to its comprehensive benefits in treating type 2 diabetes, heart failure, and chronic kidney disease, along with its relatively favorable safety profile.
Propiedades
IUPAC Name |
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JVHXJTBJCFBINQ-ADAARDCZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)C3C(C(C(C(O3)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H25ClO6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20905104 | |
| Record name | Dapagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20905104 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
408.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Dapagliflozin inhibits the sodium-glucose contransporter 2(SGLT2) which is primarily located in the proximal tubule of the nephron. SGLT2 facilitates 90% of glucose reabsorption in the kidneys and so its inhibition allows for glucose to be excreted in the urine. This excretion allows for better glycemic control and potentially weight loss in patients with type 2 diabetes mellitus. | |
| Record name | Dapagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06292 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
461432-26-8 | |
| Record name | Dapagliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=461432-26-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dapagliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0461432268 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dapagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06292 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dapagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20905104 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DAPAGLIFLOZIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1ULL0QJ8UC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of dapagliflozin?
A1: Dapagliflozin selectively inhibits SGLT2, a protein primarily located in the S1 segment of the proximal convoluted tubule in the kidneys []. SGLT2 is responsible for the majority of glucose reabsorption from the glomerular filtrate back into the bloodstream. By inhibiting SGLT2, dapagliflozin promotes urinary glucose excretion, thereby lowering blood glucose levels independently of insulin secretion or sensitivity [].
Q2: Does dapagliflozin affect other SGLT transporters in the body?
A2: Dapagliflozin demonstrates high selectivity for SGLT2 over SGLT1, another glucose transporter found in the intestines and kidneys []. This selectivity is crucial as SGLT1 inhibition can lead to gastrointestinal side effects.
Q3: How does dapagliflozin impact glucose homeostasis in the liver?
A3: While dapagliflozin primarily acts in the kidneys, research suggests it can influence hepatic glucose metabolism. Studies in obese Zucker rats show dapagliflozin increases hepatic fatty acid oxidation and ketone body formation []. This effect is attributed to the increased reliance on fatty acids as fuel in the context of dapagliflozin-induced glycosuria, mimicking a fasting state [].
Q4: Beyond its glucose-lowering effects, what other potential benefits has dapagliflozin demonstrated in preclinical studies?
A4: Preclinical studies indicate dapagliflozin may offer benefits beyond glycemic control. These include:
- Cardioprotection: Research suggests dapagliflozin may protect against doxorubicin and trastuzumab-induced cardiotoxicity by reducing oxidative stress and inflammation in cardiomyocytes [, ].
- Neuroprotection: Studies in diabetic mice indicate dapagliflozin may protect retinal neural and vascular function by reducing inflammation and improving glycemic control [].
- Renal Protection: While some research suggests dapagliflozin may increase the risk of certain renal events [], other studies indicate potential benefits. For instance, dapagliflozin has been shown to attenuate contrast-induced acute kidney injury by regulating the HIF-1α/HE4/NF-κB pathway [].
- Testicular Function: Dapagliflozin has been observed to improve testicular structure and sperm quality in diabetic mice, potentially through the activation of the GLP-1R/PI3K/Akt signaling pathway [].
Q5: What is the molecular formula and weight of dapagliflozin?
A5: The molecular formula of dapagliflozin propanediol monohydrate is C21H25ClO6 • C3H8O2, and its molecular weight is 480.9 g/mol. Information regarding the molecular formula and weight of dapagliflozin formate is unavailable in the provided abstracts.
Q6: How is dapagliflozin metabolized in the body?
A6: Dapagliflozin is primarily metabolized by UGT1A1, an enzyme involved in glucuronidation, to an inactive metabolite, dapagliflozin 3-O-glucuronide [].
Q7: What is the primary route of elimination for dapagliflozin?
A7: Dapagliflozin is mainly eliminated through the kidneys with a mean plasma terminal half-life of 12.9 hours after a single 10 mg oral dose [].
Q8: Has research investigated the pharmacokinetic profile of dapagliflozin formate (DAP-FOR)?
A8: A study comparing dapagliflozin formate (DAP-FOR) to dapagliflozin propanediol monohydrate (DAP-PDH) in healthy subjects showed DAP-FOR is rapidly converted into dapagliflozin, resulting in very low DAP-FOR exposure []. This rapid conversion leads to comparable pharmacokinetic profiles of dapagliflozin between the two formulations.
Q9: Are there specific patient populations where dapagliflozin's efficacy may be influenced?
A9: Research suggests the efficacy of dapagliflozin may vary depending on patient characteristics:
- Baseline Blood Pressure: Dapagliflozin's benefits on heart failure and renal outcomes were consistent across different baseline systolic blood pressure categories, suggesting its efficacy is independent of blood pressure levels [].
- Kidney Function: In patients with heart failure and mildly reduced or preserved ejection fraction, dapagliflozin's benefits on the primary outcome of cardiovascular death or worsening heart failure were consistent regardless of baseline kidney function [].
- Diet: The efficacy of dapagliflozin might be attenuated in individuals consuming a low-carbohydrate diet compared to a normal carbohydrate diet []. Studies in mice suggest this difference may be attributed to variations in liver metabolism between the two dietary groups.
Q10: What are some promising areas of ongoing research related to dapagliflozin?
A13: * Biomarkers: Identifying biomarkers to predict treatment response, monitor efficacy, and assess the risk of adverse events is crucial. Ongoing research focuses on discovering reliable biomarkers for dapagliflozin therapy [, ].* Drug Delivery: Developing targeted drug delivery strategies could potentially enhance dapagliflozin's efficacy and minimize off-target effects [].* Combination Therapies: Exploring the synergistic potential of dapagliflozin with other antidiabetic agents, such as GLP-1 receptor agonists [], holds promise for optimizing glycemic control and patient outcomes.
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![N-(tert-butyl)-N'-[4-(1H-imidazol-4-yl)phenyl]imidoformamide](/img/structure/B1669729.png)
![ethyl 3-[[2-[[4-[(Z)-N'-hexoxycarbonylcarbamimidoyl]anilino]methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoate;methanesulfonic acid](/img/structure/B1669742.png)