Dapagliflozin
概要
説明
Dapagliflozin is a medication primarily used to treat type 2 diabetes mellitus. It belongs to the class of sodium-glucose co-transporter 2 inhibitors. By inhibiting this transporter, this compound reduces glucose reabsorption in the kidneys, leading to increased glucose excretion in the urine. This helps in lowering blood glucose levels. Additionally, this compound is used to treat heart failure and chronic kidney disease .
作用機序
ダパグリフロジンは、近位尿細管のナトリウム-グルコース共輸送体2を阻害することにより作用します。このトランスポーターは、尿細管腔からのグルコースの再吸収を担当しています。このトランスポーターを阻害することにより、ダパグリフロジンはグルコースの再吸収を減らし、尿中へのグルコース排泄を増加させます。これは、血糖値を下げる結果になります。 さらに、ダパグリフロジンはナトリウムの再吸収を減らし、これは心臓の前負荷と後負荷の両方を下げ、交感神経系の活動を抑制するなど、いくつかの生理学的機能に影響を与える可能性があります .
類似の化合物:
- エンパグリフロジン
- カナグリフロジン
- イプラグリフロジン
比較:
- エンパグリフロジン: ダパグリフロジンとエンパグリフロジンの両方は、2型糖尿病の治療に使用されるナトリウム-グルコース共輸送体2阻害剤です。 ダパグリフロジンは、エンパグリフロジンと比較して心不全による入院のリスクが高いことが示されています .
- カナグリフロジン: ダパグリフロジンと同様に、カナグリフロジンは2型糖尿病の治療に使用され、心血管イベントを減らすという追加の利点があります。カナグリフロジンは、下肢切断のリスクが高いことが示されています。
- イプラグリフロジン: グルコース低下効果が類似した別のナトリウム-グルコース共輸送体2阻害剤です。 ダパグリフロジンは、性器感染症に関してより好ましい安全性プロファイルを持っています .
ダパグリフロジンは、2型糖尿病、心不全、慢性腎臓病の治療における包括的な利点と、比較的良好な安全性プロファイルによって際立っています。
生化学分析
Biochemical Properties
Dapagliflozin plays a crucial role in biochemical reactions by inhibiting the SGLT2 protein, which is predominantly expressed in the proximal renal tubules. This inhibition prevents glucose reabsorption, leading to its excretion through urine. This compound interacts with various enzymes and proteins, including sex hormone-binding globulin, transferrin receptor protein 1, disintegrin, metalloprotease-like decysin-1, and apolipoprotein A-IV, increasing their levels. Conversely, it decreases the levels of complement C3, fibronectin, afamin, attractin, xanthine, and uric acid .
Cellular Effects
This compound influences various cell types and cellular processes. It enhances insulin sensitivity in hepatic and adipose tissues, leading to improved glucose metabolism. This compound also affects cell signaling pathways, such as the MYD88, NLRP3 complex, Leukotrienes/Interleukin 6 axis, and mTORC1/Fox01/3a mediated apoptosis . These interactions result in changes in gene expression and cellular metabolism, contributing to its therapeutic effects.
Molecular Mechanism
At the molecular level, this compound exerts its effects by binding to the SGLT2 protein, inhibiting its function, and preventing glucose reabsorption in the kidneys. This inhibition leads to increased urinary glucose excretion, mimicking caloric restriction and promoting fat oxidation . This compound also influences gene expression by modulating the activity of various transcription factors and signaling pathways, contributing to its beneficial effects on glucose metabolism and insulin sensitivity.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound change over time. Studies have shown that this compound treatment for 12 weeks significantly decreases HbA1c, BMI, and HOMA-IR in patients with type 2 diabetes . The stability and degradation of this compound in vitro and in vivo settings are crucial for understanding its long-term effects on cellular function. This compound has been shown to maintain its efficacy over extended periods, with consistent improvements in metabolic parameters.
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At therapeutic doses, this compound effectively reduces glucose levels and improves insulin sensitivity. At higher doses, it may cause adverse effects, such as dehydration and electrolyte imbalances. Studies have identified threshold effects, where the benefits of this compound plateau beyond a certain dosage, emphasizing the importance of optimal dosing for therapeutic efficacy .
Metabolic Pathways
This compound is involved in several metabolic pathways, including glycolysis, gluconeogenesis, and the pentose phosphate pathway. It interacts with enzymes such as UGT1A9, which metabolizes this compound to its major inactive metabolite 3-O-glucuronide . These interactions influence metabolic flux and metabolite levels, contributing to the overall metabolic effects of this compound.
Transport and Distribution
This compound is transported and distributed within cells and tissues through various transporters and binding proteins. It is primarily localized in the kidneys, where it exerts its glucose-lowering effects by inhibiting SGLT2. The distribution of this compound within other tissues, such as the liver and adipose tissue, also contributes to its metabolic effects .
Subcellular Localization
The subcellular localization of this compound is primarily in the proximal renal tubules, where it inhibits SGLT2. This localization is crucial for its activity and function, as it directly affects glucose reabsorption in the kidneys. This compound may also undergo post-translational modifications that influence its targeting to specific compartments or organelles, further modulating its therapeutic effects .
準備方法
合成経路と反応条件: ダパグリフロジンの合成には、いくつかの重要なステップが含まれます。
グリニャール試薬の調製: 最初の原料である1-クロロ-2-(4-エトキシベンジル)-4-ヨードベンゼンは、ヨウ素粒を使用して開始剤としてマグネシウムと反応してグリニャール試薬を調製します。
臭素化反応: 過アセチル化糖は、酢酸溶液中の臭化水素と反応して2,3,4,6-テトラアセチルグルコサミン臭化物を調製します。
中間体の形成: グリニャール試薬をメチルベンゼン/テトラヒドロフラン溶液に加え、続いて希土類触媒と2,3,4,6-テトラアセチルグルコサミン臭化物を加えると、中間体が生成されます。
加水分解と再結晶: 中間体は、水酸化ナトリウムのテトラヒドロフラン/エタノール/水溶液中でアルカリ加水分解を受け、その後再結晶してダパグリフロジンを得ます.
工業生産方法: ダパグリフロジンの工業生産は、同様の合成経路に従いますが、より大規模に行われ、高純度と収率が確保されます。 このプロセスには、不純物を最小限に抑え、最終製品の一貫性を確保するための厳しい品質管理措置が含まれます .
反応の種類:
酸化: ダパグリフロジンは酸化反応を起こすことができ、オキソダパグリフロジンなどの代謝産物の生成につながります。
還元: 還元反応は、ダパグリフロジンをその還元形に変換できます。
置換: 置換反応は、ダパグリフロジン分子のさまざまな位置で起こることができ、さまざまな誘導体の生成につながります。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムがあります。
還元: 水素化ホウ素ナトリウムや水素化アルミニウムリチウムなどの還元剤が使用されます。
置換: 置換反応では、さまざまなハロゲン化剤と求核剤が使用されます。
主な製品:
ベンジルヒドロキシダパグリフロジン: 水酸化によって形成されます。
オキソダパグリフロジン: 酸化によって形成されます。
デスエチルダパグリフロジン: 脱エチル化によって形成されます.
4. 科学研究の応用
ダパグリフロジンは、科学研究において幅広い応用範囲を持っています。
科学的研究の応用
Dapagliflozin has a wide range of scientific research applications:
Chemistry: Used as a model compound to study the behavior of sodium-glucose co-transporter 2 inhibitors.
Biology: Investigated for its effects on glucose metabolism and renal function.
Medicine: Extensively studied for its therapeutic effects in treating type 2 diabetes, heart failure, and chronic kidney disease. .
類似化合物との比較
- Empagliflozin
- Canagliflozin
- Ipragliflozin
Comparison:
- Empagliflozin: Both dapagliflozin and empagliflozin are sodium-glucose co-transporter 2 inhibitors used to treat type 2 diabetes. this compound has shown a higher risk of hospitalization for heart failure compared to empagliflozin .
- Canagliflozin: Similar to this compound, canagliflozin is used to treat type 2 diabetes and has additional benefits in reducing cardiovascular events. canagliflozin has been associated with a higher risk of lower limb amputations.
- Ipragliflozin: Another sodium-glucose co-transporter 2 inhibitor with similar glucose-lowering effects. this compound has a more favorable safety profile in terms of genital infections .
This compound stands out due to its comprehensive benefits in treating type 2 diabetes, heart failure, and chronic kidney disease, along with its relatively favorable safety profile.
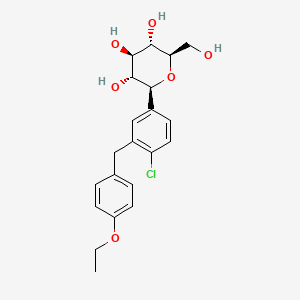
特性
IUPAC Name |
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JVHXJTBJCFBINQ-ADAARDCZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)C3C(C(C(C(O3)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H25ClO6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20905104 | |
| Record name | Dapagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20905104 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
408.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Dapagliflozin inhibits the sodium-glucose contransporter 2(SGLT2) which is primarily located in the proximal tubule of the nephron. SGLT2 facilitates 90% of glucose reabsorption in the kidneys and so its inhibition allows for glucose to be excreted in the urine. This excretion allows for better glycemic control and potentially weight loss in patients with type 2 diabetes mellitus. | |
| Record name | Dapagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06292 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
461432-26-8 | |
| Record name | Dapagliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=461432-26-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dapagliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0461432268 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dapagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06292 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dapagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20905104 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DAPAGLIFLOZIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1ULL0QJ8UC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of dapagliflozin?
A1: this compound selectively inhibits SGLT2, a protein primarily located in the S1 segment of the proximal convoluted tubule in the kidneys []. SGLT2 is responsible for the majority of glucose reabsorption from the glomerular filtrate back into the bloodstream. By inhibiting SGLT2, this compound promotes urinary glucose excretion, thereby lowering blood glucose levels independently of insulin secretion or sensitivity [].
Q2: Does this compound affect other SGLT transporters in the body?
A2: this compound demonstrates high selectivity for SGLT2 over SGLT1, another glucose transporter found in the intestines and kidneys []. This selectivity is crucial as SGLT1 inhibition can lead to gastrointestinal side effects.
Q3: How does this compound impact glucose homeostasis in the liver?
A3: While this compound primarily acts in the kidneys, research suggests it can influence hepatic glucose metabolism. Studies in obese Zucker rats show this compound increases hepatic fatty acid oxidation and ketone body formation []. This effect is attributed to the increased reliance on fatty acids as fuel in the context of this compound-induced glycosuria, mimicking a fasting state [].
Q4: Beyond its glucose-lowering effects, what other potential benefits has this compound demonstrated in preclinical studies?
A4: Preclinical studies indicate this compound may offer benefits beyond glycemic control. These include:
- Cardioprotection: Research suggests this compound may protect against doxorubicin and trastuzumab-induced cardiotoxicity by reducing oxidative stress and inflammation in cardiomyocytes [, ].
- Neuroprotection: Studies in diabetic mice indicate this compound may protect retinal neural and vascular function by reducing inflammation and improving glycemic control [].
- Renal Protection: While some research suggests this compound may increase the risk of certain renal events [], other studies indicate potential benefits. For instance, this compound has been shown to attenuate contrast-induced acute kidney injury by regulating the HIF-1α/HE4/NF-κB pathway [].
- Testicular Function: this compound has been observed to improve testicular structure and sperm quality in diabetic mice, potentially through the activation of the GLP-1R/PI3K/Akt signaling pathway [].
Q5: What is the molecular formula and weight of this compound?
A5: The molecular formula of this compound propanediol monohydrate is C21H25ClO6 • C3H8O2, and its molecular weight is 480.9 g/mol. Information regarding the molecular formula and weight of this compound formate is unavailable in the provided abstracts.
Q6: How is this compound metabolized in the body?
A6: this compound is primarily metabolized by UGT1A1, an enzyme involved in glucuronidation, to an inactive metabolite, this compound 3-O-glucuronide [].
Q7: What is the primary route of elimination for this compound?
A7: this compound is mainly eliminated through the kidneys with a mean plasma terminal half-life of 12.9 hours after a single 10 mg oral dose [].
Q8: Has research investigated the pharmacokinetic profile of this compound formate (DAP-FOR)?
A8: A study comparing this compound formate (DAP-FOR) to this compound propanediol monohydrate (DAP-PDH) in healthy subjects showed DAP-FOR is rapidly converted into this compound, resulting in very low DAP-FOR exposure []. This rapid conversion leads to comparable pharmacokinetic profiles of this compound between the two formulations.
Q9: Are there specific patient populations where this compound's efficacy may be influenced?
A9: Research suggests the efficacy of this compound may vary depending on patient characteristics:
- Baseline Blood Pressure: this compound's benefits on heart failure and renal outcomes were consistent across different baseline systolic blood pressure categories, suggesting its efficacy is independent of blood pressure levels [].
- Kidney Function: In patients with heart failure and mildly reduced or preserved ejection fraction, this compound's benefits on the primary outcome of cardiovascular death or worsening heart failure were consistent regardless of baseline kidney function [].
- Diet: The efficacy of this compound might be attenuated in individuals consuming a low-carbohydrate diet compared to a normal carbohydrate diet []. Studies in mice suggest this difference may be attributed to variations in liver metabolism between the two dietary groups.
Q10: What are some promising areas of ongoing research related to this compound?
A13: * Biomarkers: Identifying biomarkers to predict treatment response, monitor efficacy, and assess the risk of adverse events is crucial. Ongoing research focuses on discovering reliable biomarkers for this compound therapy [, ].* Drug Delivery: Developing targeted drug delivery strategies could potentially enhance this compound's efficacy and minimize off-target effects [].* Combination Therapies: Exploring the synergistic potential of this compound with other antidiabetic agents, such as GLP-1 receptor agonists [], holds promise for optimizing glycemic control and patient outcomes.
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![N-(tert-butyl)-N'-[4-(1H-imidazol-4-yl)phenyl]imidoformamide](/img/structure/B1669729.png)