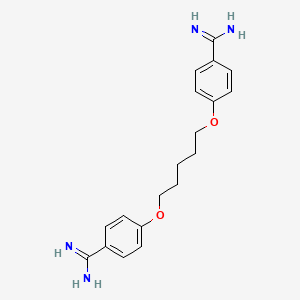
Pentamidina
Descripción general
Descripción
La pentamidina es un medicamento antimicrobiano que se utiliza principalmente para tratar la tripanosomiasis africana, la leishmaniasis, las infecciones por Balamuthia, la babesiosis y para prevenir y tratar la neumonía por Pneumocystis en individuos con sistemas inmunitarios comprometidos . Pertenece a la familia de medicamentos de diamidinas aromáticas y se utiliza en medicina desde 1937 . La this compound está disponible como medicamento genérico y está incluida en la Lista de medicamentos esenciales de la Organización Mundial de la Salud .
Mecanismo De Acción
El mecanismo de acción exacto de la pentamidina no se comprende completamente. Se cree que interfiere con el metabolismo nuclear al inhibir la síntesis de ADN, ARN, fosfolípidos y proteínas . La this compound se une a los ácidos nucleicos a través de sus iones amidinio cargados positivamente, que la dirigen hacia la columna vertebral de fosfato cargada negativamente del ADN y el ARN . Esta interacción interrumpe el funcionamiento normal de los ácidos nucleicos, lo que lleva a los efectos antimicrobianos del fármaco .
Compuestos similares:
Dapsona: Se utiliza para el tratamiento de la dermatitis herpetiforme, la lepra y otras afecciones de la piel.
Sulfametoxazol/Trimetoprima: Una combinación antibiótica que se utiliza para tratar diversas infecciones bacterianas.
Comparación: La this compound es única en su capacidad para tratar una amplia gama de infecciones protozoarias, incluidas la tripanosomiasis africana y la leishmaniasis, que no suelen tratarse con dapsona o sulfametoxazol/trimetoprima . Además, el mecanismo de acción de la this compound, que implica la inhibición de la síntesis de ácidos nucleicos, la diferencia de otros agentes antimicrobianos .
Aplicaciones Científicas De Investigación
La pentamidina tiene una amplia gama de aplicaciones de investigación científica:
Análisis Bioquímico
Biochemical Properties
Pentamidine interacts with various biomolecules within the cell. It is thought to interfere with nuclear metabolism, inhibiting the synthesis of DNA, RNA, phospholipids, and proteins . This disruption of essential biochemical reactions is believed to contribute to its antifungal and antiprotozoal effects .
Cellular Effects
Pentamidine has significant effects on various types of cells and cellular processes. It is known to cause diabetes mellitus, central nervous system damage, and other toxic effects . In addition, it has been found to reduce mitochondrial protein abundance and trigger progressive loss of kinetoplast DNA and disruption of mitochondrial membrane potential .
Molecular Mechanism
It is believed to exert its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . For instance, it has been found to inhibit mitosis and cytokinesis .
Temporal Effects in Laboratory Settings
Over time, Pentamidine has been observed to have various effects in laboratory settings. For instance, it has been found to cause hypoglycemia and nephrotoxicity in up to 27% and 25% of treatment courses, respectively .
Dosage Effects in Animal Models
The effects of Pentamidine vary with different dosages in animal models. For instance, it has been found to effectively decrease the burden of Chlamydia trachomatis upon local or systemic application in mice .
Metabolic Pathways
Pentamidine is involved in various metabolic pathways. It is thought to change the metabolism of host cells, impairing chlamydia growth .
Transport and Distribution
Pentamidine is transported and distributed within cells and tissues via various mechanisms. It has been found to be involved in pentamidine transport at the human and mouse blood-brain barrier (BBB) via the organic cation transporter 1 (OCT1) .
Subcellular Localization
Pentamidine localizes in various subcellular compartments. It has been found to localize in parasite nuclei and kDNA, with greater intensity in the latter structure . Furthermore, it also concentrates in non-DNA-containing cytoplasmic organelles, possibly acidocalcisomes .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La pentamidina se puede sintetizar mediante un proceso de varios pasos que implica la reacción de 4,4'-dihidroxi-benzofenona con bromuro de pentametileno para formar 4,4'-bis(pentametilenodioxi)benzofenona. Este intermedio se convierte luego en 4,4'-bis(pentametilenodioxi)dibenzamidina mediante una reacción con cloruro de amonio y cianuro de sodio .
Métodos de producción industrial: En entornos industriales, la this compound se prepara típicamente reconstituyendo el medicamento con agua para inyección. La solución reconstituida se diluye aún más antes de la administración. Para la infusión intravenosa, el medicamento se administra mediante un dispositivo de infusión controlado electrónicamente durante un período de 60 a 120 minutos .
Análisis De Reacciones Químicas
Tipos de reacciones: La pentamidina sufre varias reacciones químicas, incluidas reacciones de reducción y sustitución. Una reacción notable es su reducción electroquímica, que se ha estudiado utilizando voltametría cíclica .
Reactivos y condiciones comunes:
Reducción: En un tampón de fosfato de pH 8.5, la this compound produce una onda de reducción sensible a un potencial de -1.56 V (vs.
Sustitución: La this compound puede sufrir reacciones de sustitución con nucleófilos, lo que lleva a la formación de varios derivados.
Productos principales: Los principales productos formados a partir de estas reacciones incluyen formas reducidas de this compound y sus diversos derivados sustituidos .
Comparación Con Compuestos Similares
Dapsone: Used for the treatment of dermatitis herpetiformis, leprosy, and other skin conditions.
Sulfamethoxazole/Trimethoprim: An antibiotic combination used to treat various bacterial infections.
Comparison: Pentamidine is unique in its ability to treat a wide range of protozoal infections, including African trypanosomiasis and leishmaniasis, which are not typically treated with dapsone or sulfamethoxazole/trimethoprim . Additionally, pentamidine’s mechanism of action, involving the inhibition of nucleic acid synthesis, sets it apart from other antimicrobial agents .
Propiedades
IUPAC Name |
4-[5-(4-carbamimidoylphenoxy)pentoxy]benzenecarboximidamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H24N4O2/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XDRYMKDFEDOLFX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=CC=C1C(=N)N)OCCCCCOC2=CC=C(C=C2)C(=N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H24N4O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7023431 | |
| Record name | Pentamidine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023431 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
340.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Complete, Mol wt: 592.69. Hygroscopic, very bitter crystals, mp approx 180 °C. Slight butyric odor. Sol in water (approx 1 in 10 at 25 °C, approx 1 in 4 at 100 °C); sol in glycerol, more readily on warming; slightly sol in alcohol. Insol in ether, acetone, chloroform, liq petr. pH of a 5% w/v soln in water: 4.5 to 6.5. /Isethioante/, 2.36e-02 g/L | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The mode of action of pentamidine is not fully understood. It is thought that the drug interferes with nuclear metabolism producing inhibition of the synthesis of DNA, RNA, phospholipids, and proteins., ... Up to now, it has been thought that therapeutic compounds causing QT prolongation are associated with direct block of the cardiac potassium channel human ether a-go-go-related gene (hERG), which encodes the alpha subunit of cardiac I(Kr) currents. /The authors/ show that pentamidine has no acute effects on currents produced by hERG, KvLQT1/mink, Kv4.3, or SCNA5. Cardiac calcium currents and the guinea pig cardiac action potential were also not affected. After overnight exposure, however, pentamidine reduced hERG currents and inhibited trafficking and maturation of hERG with IC(50) values of 5 to 8 uM similar to therapeutic concentrations. Surface expression determined in a chemiluminescence assay was reduced on exposure to 10, 30, and 100 uM pentamidine by about 30, 40, and 70%, respectively. These effects were specific for hERG since expression of hKv1.5, KvLQT1/minK, and Kv4.3 was not altered. In isolated guinea pig ventricular myocytes, 10 uM pentamidine prolonged action potential duration APD(90) from 374.3 or + or - 57.1 to 893.9 + or - 86.2 ms on overnight incubation. I(Kr) tail current density was reduced from 0.61 + or - 0.09 to 0.39 + or - 0.04 pA/pF. /The authors/ conclude that pentamidine prolongs the cardiac action potential by block of hERG trafficking and reduction of the number of functional hERG channels at the cell surface. /The authors/ propose that pentamidine, like arsenic trioxide, produces QT prolongation and torsades de pointes in patients by inhibition of hERG trafficking., ... Inhibition in vitro of trypanosomal mitochondrial topoisomerase II and plasma Ca+2, Mg+2-ATPase also has been reported ... Pentamidine promotes linearization of trypanosome kinetoplast DNA, consistent with its being a type II topoisomerase inhibitor ... The drug also inhibits ATP-dependent topoisomerases in extracts of Pneumocystis carinii ..., Not clearly defined; pentamidine may interfere with incorporation of nucleotides into RNA and DNA and inhibit oxidative phosphorylation and biosynthesis of DNA, RNA, protein, and phospholipid; may also interfere with folate transformation., ... The cytotoxic properties of pentamidine isethionate (2) towards the promastigotes of the protozoan parasite Leishmania infantum /was determined/. The leishmanicidal activity of 2 was 60 times higher after 72 hr of incubation than that of cisplatin. The pentamidine salt 2 induced a higher amount of programmed cell death (PCD) than cisplatin, which is associated with inhibition of DNA synthesis and cell-cycle arrest in the G2/M phase. Circular dichroism (CD) data indicate that binding of 2 to calf-thymus DNA (CT-DNA) induces conformational changes in the DNA double helix, consistent with a B-->A transition. Moreover, the interaction of 2 with ubiquitin led to a 6% increase in the beta-sheet content of the protein as observed by CD spectroscopy. Fluorescence-spectroscopy studies agreed with the CD data, showing that the pentamidine portion of 2 induces a significant decrease in the fluorescence of the Ub residues Phe4 and Phe45 located on the beta-cluster of the molecule, but not of Tyr59 on the alpha-cluster. These data indicate that pentamidine specifically modifies the beta-cluster, i.e., the 'basic face' of ubiquitin. ... /The/ results suggest that the biochemical mechanism of action of pentamidine may be a consequence of its dual binding to DNA and proteins., In this work pentamidine is shown to exhibit characteristics of a cationic uncoupler of oxidative phosphorylation in isolated rat liver mitochondria: it released respiratory control, enhanced the latent ATPase activity, and released the inhibition of State 3 respiration by oligomycin. Maximal stimulation of respiration and ATPase activity was observed at a concentration of pentamidine of 200-300 microM. Higher concentrations had an inhibitory effect on mitochondrial respiration. As it happens with other cationic uncouplers, the uncoupling effect of pentamidine required inorganic phosphate. Pentamidine-induced uncoupling of oxidative phosphorylation was accompanied by an efflux of Ca2+ from the mitochondria and partial collapse of the mitochondrial membrane potential. | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystallizes as colorless plates from water | |
CAS No. |
100-33-4 | |
| Record name | Pentamidine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=100-33-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pentamidine [INN:BAN:DCF] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000100334 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | pentamidine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=9921 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pentamidine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023431 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Pentamidine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.002.583 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PENTAMIDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/673LC5J4LQ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
Decomposes at 186 °C, 186.0 °C (decomposes) | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.

![[(19S)-19-ethyl-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaen-19-yl] (2S)-2-[[2-[2-[2-[[(2S)-1-[[(19S)-19-ethyl-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaen-19-yl]oxy]-1-oxopropan-2-yl]amino]-2-oxoethoxy]ethoxy]acetyl]amino]propanoate](/img/structure/B1679208.png)