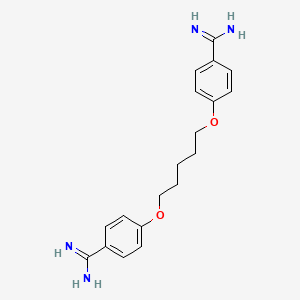
喷他脒
概述
描述
作用机制
彭他胺的精确作用机制尚不清楚。 据信它通过抑制 DNA、RNA、磷脂和蛋白质的合成来干扰核代谢 . 彭他胺通过其带正电荷的脒离子与核酸结合,将其引导到 DNA 和 RNA 的带负电荷的磷酸骨架 . 这种相互作用破坏了核酸的正常功能,从而导致药物的抗菌作用 .
类似化合物:
达普森: 用于治疗疱疹样皮炎、麻风病和其他皮肤病.
磺胺甲噁唑/甲氧苄啶: 一种抗生素组合,用于治疗各种细菌感染.
比较: 彭他胺在治疗多种原生动物感染(包括非洲锥虫病和利什曼病)方面独一无二,这些感染通常不使用达普森或磺胺甲噁唑/甲氧苄啶治疗 . 此外,彭他胺的作用机制涉及抑制核酸合成,使其有别于其他抗菌剂 .
科学研究应用
彭他胺具有广泛的科学研究应用:
生化分析
Biochemical Properties
Pentamidine interacts with various biomolecules within the cell. It is thought to interfere with nuclear metabolism, inhibiting the synthesis of DNA, RNA, phospholipids, and proteins . This disruption of essential biochemical reactions is believed to contribute to its antifungal and antiprotozoal effects .
Cellular Effects
Pentamidine has significant effects on various types of cells and cellular processes. It is known to cause diabetes mellitus, central nervous system damage, and other toxic effects . In addition, it has been found to reduce mitochondrial protein abundance and trigger progressive loss of kinetoplast DNA and disruption of mitochondrial membrane potential .
Molecular Mechanism
It is believed to exert its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . For instance, it has been found to inhibit mitosis and cytokinesis .
Temporal Effects in Laboratory Settings
Over time, Pentamidine has been observed to have various effects in laboratory settings. For instance, it has been found to cause hypoglycemia and nephrotoxicity in up to 27% and 25% of treatment courses, respectively .
Dosage Effects in Animal Models
The effects of Pentamidine vary with different dosages in animal models. For instance, it has been found to effectively decrease the burden of Chlamydia trachomatis upon local or systemic application in mice .
Metabolic Pathways
Pentamidine is involved in various metabolic pathways. It is thought to change the metabolism of host cells, impairing chlamydia growth .
Transport and Distribution
Pentamidine is transported and distributed within cells and tissues via various mechanisms. It has been found to be involved in pentamidine transport at the human and mouse blood-brain barrier (BBB) via the organic cation transporter 1 (OCT1) .
Subcellular Localization
Pentamidine localizes in various subcellular compartments. It has been found to localize in parasite nuclei and kDNA, with greater intensity in the latter structure . Furthermore, it also concentrates in non-DNA-containing cytoplasmic organelles, possibly acidocalcisomes .
准备方法
合成路线和反应条件: 彭他胺可以通过多步合成法制备,涉及4,4’-二羟基苯乙酮与五亚甲基溴化物反应生成4,4’-双(五亚甲基二氧)苯乙酮。 该中间体随后与氯化铵和氰化钠反应生成4,4’-双(五亚甲基二氧)二苯甲脒 .
工业生产方法: 在工业环境中,彭他胺通常通过使用注射用水重构成药剂。然后在给药前进一步稀释重构溶液。 对于静脉输液,药物使用电子控制的输液装置在 60 到 120 分钟内输注 .
化学反应分析
反应类型: 彭他胺会发生多种化学反应,包括还原反应和取代反应。 值得注意的是其电化学还原反应,已通过循环伏安法进行研究 .
常用试剂和条件:
主要产物: 这些反应产生的主要产物包括彭他胺的还原形式及其各种取代衍生物 .
相似化合物的比较
Dapsone: Used for the treatment of dermatitis herpetiformis, leprosy, and other skin conditions.
Sulfamethoxazole/Trimethoprim: An antibiotic combination used to treat various bacterial infections.
Comparison: Pentamidine is unique in its ability to treat a wide range of protozoal infections, including African trypanosomiasis and leishmaniasis, which are not typically treated with dapsone or sulfamethoxazole/trimethoprim . Additionally, pentamidine’s mechanism of action, involving the inhibition of nucleic acid synthesis, sets it apart from other antimicrobial agents .
属性
IUPAC Name |
4-[5-(4-carbamimidoylphenoxy)pentoxy]benzenecarboximidamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H24N4O2/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XDRYMKDFEDOLFX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=CC=C1C(=N)N)OCCCCCOC2=CC=C(C=C2)C(=N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H24N4O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7023431 | |
| Record name | Pentamidine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023431 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
340.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Complete, Mol wt: 592.69. Hygroscopic, very bitter crystals, mp approx 180 °C. Slight butyric odor. Sol in water (approx 1 in 10 at 25 °C, approx 1 in 4 at 100 °C); sol in glycerol, more readily on warming; slightly sol in alcohol. Insol in ether, acetone, chloroform, liq petr. pH of a 5% w/v soln in water: 4.5 to 6.5. /Isethioante/, 2.36e-02 g/L | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The mode of action of pentamidine is not fully understood. It is thought that the drug interferes with nuclear metabolism producing inhibition of the synthesis of DNA, RNA, phospholipids, and proteins., ... Up to now, it has been thought that therapeutic compounds causing QT prolongation are associated with direct block of the cardiac potassium channel human ether a-go-go-related gene (hERG), which encodes the alpha subunit of cardiac I(Kr) currents. /The authors/ show that pentamidine has no acute effects on currents produced by hERG, KvLQT1/mink, Kv4.3, or SCNA5. Cardiac calcium currents and the guinea pig cardiac action potential were also not affected. After overnight exposure, however, pentamidine reduced hERG currents and inhibited trafficking and maturation of hERG with IC(50) values of 5 to 8 uM similar to therapeutic concentrations. Surface expression determined in a chemiluminescence assay was reduced on exposure to 10, 30, and 100 uM pentamidine by about 30, 40, and 70%, respectively. These effects were specific for hERG since expression of hKv1.5, KvLQT1/minK, and Kv4.3 was not altered. In isolated guinea pig ventricular myocytes, 10 uM pentamidine prolonged action potential duration APD(90) from 374.3 or + or - 57.1 to 893.9 + or - 86.2 ms on overnight incubation. I(Kr) tail current density was reduced from 0.61 + or - 0.09 to 0.39 + or - 0.04 pA/pF. /The authors/ conclude that pentamidine prolongs the cardiac action potential by block of hERG trafficking and reduction of the number of functional hERG channels at the cell surface. /The authors/ propose that pentamidine, like arsenic trioxide, produces QT prolongation and torsades de pointes in patients by inhibition of hERG trafficking., ... Inhibition in vitro of trypanosomal mitochondrial topoisomerase II and plasma Ca+2, Mg+2-ATPase also has been reported ... Pentamidine promotes linearization of trypanosome kinetoplast DNA, consistent with its being a type II topoisomerase inhibitor ... The drug also inhibits ATP-dependent topoisomerases in extracts of Pneumocystis carinii ..., Not clearly defined; pentamidine may interfere with incorporation of nucleotides into RNA and DNA and inhibit oxidative phosphorylation and biosynthesis of DNA, RNA, protein, and phospholipid; may also interfere with folate transformation., ... The cytotoxic properties of pentamidine isethionate (2) towards the promastigotes of the protozoan parasite Leishmania infantum /was determined/. The leishmanicidal activity of 2 was 60 times higher after 72 hr of incubation than that of cisplatin. The pentamidine salt 2 induced a higher amount of programmed cell death (PCD) than cisplatin, which is associated with inhibition of DNA synthesis and cell-cycle arrest in the G2/M phase. Circular dichroism (CD) data indicate that binding of 2 to calf-thymus DNA (CT-DNA) induces conformational changes in the DNA double helix, consistent with a B-->A transition. Moreover, the interaction of 2 with ubiquitin led to a 6% increase in the beta-sheet content of the protein as observed by CD spectroscopy. Fluorescence-spectroscopy studies agreed with the CD data, showing that the pentamidine portion of 2 induces a significant decrease in the fluorescence of the Ub residues Phe4 and Phe45 located on the beta-cluster of the molecule, but not of Tyr59 on the alpha-cluster. These data indicate that pentamidine specifically modifies the beta-cluster, i.e., the 'basic face' of ubiquitin. ... /The/ results suggest that the biochemical mechanism of action of pentamidine may be a consequence of its dual binding to DNA and proteins., In this work pentamidine is shown to exhibit characteristics of a cationic uncoupler of oxidative phosphorylation in isolated rat liver mitochondria: it released respiratory control, enhanced the latent ATPase activity, and released the inhibition of State 3 respiration by oligomycin. Maximal stimulation of respiration and ATPase activity was observed at a concentration of pentamidine of 200-300 microM. Higher concentrations had an inhibitory effect on mitochondrial respiration. As it happens with other cationic uncouplers, the uncoupling effect of pentamidine required inorganic phosphate. Pentamidine-induced uncoupling of oxidative phosphorylation was accompanied by an efflux of Ca2+ from the mitochondria and partial collapse of the mitochondrial membrane potential. | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystallizes as colorless plates from water | |
CAS No. |
100-33-4 | |
| Record name | Pentamidine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=100-33-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pentamidine [INN:BAN:DCF] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000100334 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | pentamidine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=9921 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pentamidine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023431 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Pentamidine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.002.583 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PENTAMIDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/673LC5J4LQ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
Decomposes at 186 °C, 186.0 °C (decomposes) | |
| Record name | Pentamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00738 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PENTAMIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7474 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pentamidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014876 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。

![[(19S)-19-ethyl-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaen-19-yl] (2S)-2-[[2-[2-[2-[[(2S)-1-[[(19S)-19-ethyl-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaen-19-yl]oxy]-1-oxopropan-2-yl]amino]-2-oxoethoxy]ethoxy]acetyl]amino]propanoate](/img/structure/B1679208.png)