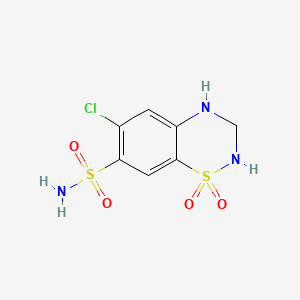
Hydrochlorothiazide
Vue d'ensemble
Description
L’hydrochlorothiazide est un diurétique thiazidique largement utilisé, principalement prescrit pour le traitement de l’hypertension artérielle (pression artérielle élevée) et de l’œdème (rétention d’eau). Il est également utilisé pour gérer des affections telles que le diabète insipide et l’acidose tubulaire rénale, et pour réduire le risque de calculs rénaux chez les personnes ayant des niveaux élevés de calcium dans leur urine . L’this compound agit en inhibant la réabsorption des ions sodium et chlorure dans les reins, ce qui entraîne une augmentation de la production d’urine et une diminution du volume sanguin .
Mécanisme D'action
L’hydrochlorothiazide exerce ses effets en inhibant le symporteur sodium-chlorure dans les tubules contournés distaux des reins. Cette inhibition empêche la réabsorption des ions sodium et chlorure, ce qui entraîne une excrétion accrue de ces ions ainsi que de l’eau. L’effet diurétique qui en résulte réduit le volume sanguin et diminue la résistance vasculaire périphérique, ce qui abaisse la pression artérielle . De plus, l’action de l’this compound sur le transport ionique peut influencer l’équilibre électrolytique et la fonction rénale .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : L’hydrochlorothiazide est synthétisé par un processus chimique en plusieurs étapes. La synthèse implique généralement la réaction de la 3-chloroaniline avec l’acide chlorosulfonique pour former la 3-chloro-4-sulfamoylaniline. Cet intermédiaire est ensuite cyclisé avec de la thiourée pour produire la structure cyclique thiazidique, ce qui donne l’this compound .
Méthodes de production industrielle : La production industrielle d’this compound implique des voies de synthèse similaires, mais à plus grande échelle. Le processus est optimisé pour un rendement et une pureté élevés, impliquant souvent des techniques avancées telles que la chromatographie liquide haute performance (CLHP) pour la purification et le contrôle de la qualité .
Analyse Des Réactions Chimiques
Types de réactions : L’hydrochlorothiazide subit diverses réactions chimiques, notamment :
Oxydation : L’this compound peut être oxydé dans des conditions spécifiques, ce qui conduit à la formation de sulfoxydes et de sulfones.
Réduction : Les réactions de réduction peuvent convertir l’this compound en ses dérivés aminés correspondants.
Substitution : Des réactions de substitution nucléophile peuvent se produire au niveau du groupe chloro, conduisant à la formation de divers dérivés substitués.
Réactifs et conditions courants :
Oxydation : Les agents oxydants courants comprennent le peroxyde d’hydrogène et le permanganate de potassium.
Réduction : Des agents réducteurs tels que l’hydrure de lithium aluminium et le borohydrure de sodium sont utilisés.
Substitution : Des nucléophiles comme les amines et les thiols sont utilisés dans les réactions de substitution.
Principaux produits :
Oxydation : Sulfoxydes et sulfones.
Réduction : Dérivés aminés.
Substitution : Dérivés thiazidiques substitués.
4. Applications de la recherche scientifique
L’this compound a un large éventail d’applications de recherche scientifique :
Chimie : Utilisé comme composé modèle dans des études sur les mécanismes diurétiques et les interactions médicamenteuses.
Biologie : Investigué pour ses effets sur le transport ionique cellulaire et l’équilibre électrolytique.
Médecine : Étudié de manière approfondie pour ses effets thérapeutiques dans l’hypertension, l’insuffisance cardiaque et les troubles rénaux.
Industrie : Utilisé dans le développement de thérapies médicamenteuses combinées et de formulations pharmaceutiques.
Applications De Recherche Scientifique
Hydrochlorothiazide has a wide range of scientific research applications:
Chemistry: Used as a model compound in studies of diuretic mechanisms and drug interactions.
Biology: Investigated for its effects on cellular ion transport and electrolyte balance.
Medicine: Extensively studied for its therapeutic effects in hypertension, heart failure, and kidney disorders.
Industry: Utilized in the development of combination drug therapies and pharmaceutical formulations.
Comparaison Avec Des Composés Similaires
L’hydrochlorothiazide fait partie de la classe des diurétiques thiazidiques, qui comprend des composés similaires tels que la chlorothiazide, la chlorthalidone et l’indapamide. Comparé à ces composés, l’this compound est connu pour sa demi-vie relativement courte et son début d’action rapide .
Composés similaires :
Chlorothiazide : Un autre diurétique thiazidique avec une demi-vie plus longue.
Chlorthalidone : Connu pour sa durée d’action plus longue et sa puissance accrue.
Indapamide : Un diurétique de type thiazidique avec des propriétés vasodilatatrices supplémentaires.
L’équilibre unique de l’this compound en termes d’efficacité, de sécurité et de rentabilité en fait un choix largement préféré pour la prise en charge de l’hypertension et de l’œdème .
Propriétés
IUPAC Name |
6-chloro-1,1-dioxo-3,4-dihydro-2H-1λ6,2,4-benzothiadiazine-7-sulfonamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JZUFKLXOESDKRF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1NC2=CC(=C(C=C2S(=O)(=O)N1)S(=O)(=O)N)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C7H8ClN3O4S2 | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | hydrochlorothiazide | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Hydrochlorothiazide | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2020713 | |
| Record name | Hydrochlorothiazide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2020713 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
297.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Crystals or white powder. (NTP, 1992), Solid | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>44.7 [ug/mL] (The mean of the results at pH 7.4), less than 0.1 mg/mL at 72.5 °F (NTP, 1992), In water, 722 mg/L at 25 °C, Soluble in ethanol at approximately 750 g/L; soluble in acetone, dilute ammonia; freely soluble in sodium hydroxide solution, n-butylamine, dimethylformamide; sparingly soluble in alcohol; insoluble in ether, chloroform, dilute mineral acids, Soluble in sodium hydroxide solution, Freely soluble in sodium hydroxide solution, in n-butylamine and in dimethylformamide; sparingly soluble in methanol; insoluble in dilute mineral acids, 0.722 mg/mL at 25 °C | |
| Record name | SID855646 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Density |
1.693 g/cu cm | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Hydrochlorothiazide is transported from the circulation into epithelial cells of the distal convoluted tubule by the organic anion transporters OAT1, OAT3, and OAT4. From these cells, hydrochlorothiazide is transported to the lumen of the tubule by multidrug resistance associated protein 4 (MRP4). Normally, sodium is reabsorbed into epithelial cells of the distal convoluted tubule and pumped into the basolateral interstitium by a sodium-potassium ATPase, creating a concentration gradient between the epithelial cell and the distal convoluted tubule that promotes the reabsorption of water. Hydrochlorothiazide acts on the proximal region of the distal convoluted tubule, inhibiting reabsorption by the sodium-chloride symporter, also known as Solute Carrier Family 12 Member 3 (SLC12A3). Inhibition of SLC12A3 reduces the magnitude of the concentration gradient between the epithelial cell and distal convoluted tubule, reducing the reabsorption of water. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Color/Form |
White, or practically white crystalline powder, White to off-white crystalline powder | |
CAS No. |
58-93-5 | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=58-93-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Hydrochlorothiazide [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000058935 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | hydrochlorothiazide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757059 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | hydrochlorothiazide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=53477 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Hydrochlorothiazide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2020713 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Hydrochlorothiazide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.367 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0J48LPH2TH | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
523 to 527 °F (NTP, 1992), 266-268, 273-275 °C, 274 °C | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Hydrochlorothiazide exert its antihypertensive effect?
A1: this compound (HCTZ) primarily acts on the distal convoluted tubule in the kidneys to inhibit sodium and chloride reabsorption. This leads to increased sodium and water excretion, thereby reducing blood volume and lowering blood pressure. [, , , ]
Q2: Does this compound have any impact on the renin-angiotensin-aldosterone system?
A2: While HCTZ's primary mechanism doesn't directly involve the renin-angiotensin-aldosterone system, its diuretic effect can lead to a compensatory increase in plasma renin activity and aldosterone levels. [] This can sometimes counteract its blood pressure-lowering effects. [, ]
Q3: Are there any studies comparing this compound with other diuretics like indapamide?
A3: Yes, a study compared the hypotensive, metabolic, and endothelial effects of indapamide-retard and HCTZ. Despite similar blood pressure-lowering effects, indapamide showed a more favorable metabolic profile, with no significant elevation of triglycerides or glucose, unlike HCTZ. []
Q4: What is the molecular formula and weight of this compound?
A4: The molecular formula of this compound is C12H11ClN3O4S2, and its molecular weight is 353.82 g/mol.
Q5: Can Raman spectroscopy be used to study this compound inclusion complexes?
A5: Yes, Raman spectroscopy has been successfully used to study the formation of inclusion complexes between this compound and β-cyclodextrin. The technique confirmed the presence of hydrogen bonds between the drug and the cyclodextrin molecule. []
Q6: What analytical methods are commonly used to quantify this compound in pharmaceutical formulations?
A6: Several analytical methods are employed to quantify HCTZ in pharmaceutical formulations, including:
- High-Performance Liquid Chromatography (HPLC): This versatile technique allows simultaneous determination of HCTZ with other antihypertensive drugs like amlodipine, valsartan, and quinapril. [, , , ]
- UV Spectrophotometry: This method offers simplicity and cost-effectiveness for HCTZ quantification, either alone or in combination with other drugs like losartan potassium. [, ]
- High-Performance Thin Layer Chromatography (HPTLC): HPTLC provides a rapid and precise alternative for analyzing HCTZ in tablet formulations, often alongside drugs like candesartan cilexetil. []
Q7: What formulation strategies are used to improve the dissolution and bioavailability of this compound?
A7: Direct powder compression using disintegrants like croscarmellose sodium (CMS-Na) and low-substituted hydroxypropyl cellulose (L-HPC) has shown promise in improving the dissolution rate of this compound dispersible tablets. []
Q8: Are there studies on the stability of this compound under various stress conditions?
A8: Yes, stability-indicating HPLC methods have been developed and validated to assess the stability of this compound under various stress conditions like acidic, basic, oxidative, thermal, and photolytic degradation. These methods ensure the accurate determination of the drug in the presence of its degradation products. [, ]
Q9: What is the duration of action of this compound?
A9: this compound typically has a duration of action of 6 to 12 hours, but its blood pressure-lowering effect may persist for longer. [, , ]
Q10: Does this compound effectively lower both systolic and diastolic blood pressure?
A10: Clinical trials have shown that HCTZ effectively reduces both systolic and diastolic blood pressure, although a higher dose might be needed for optimal control in some patients, especially when combined with other antihypertensives. [, ]
Q11: Are there any studies investigating the long-term effects of this compound on renal hemodynamics?
A11: Research indicates that long-term HCTZ treatment in essential hypertension may positively impact renal hemodynamics, potentially reversing some of the abnormalities associated with the condition. These effects go beyond simple blood pressure reduction and may involve humoral and neural mechanisms. []
Q12: What is the comparative efficacy of this compound combined with other antihypertensives?
A12: Several studies have compared HCTZ combinations with other antihypertensive regimens:
- HCTZ + Ramipril: This combination demonstrated superior antihypertensive effects compared to HCTZ alone, particularly on nocturnal blood pressure, with significant reductions in plasma angiotensin II and aldosterone levels. []
- HCTZ + Amlodipine: This combination, in a fixed-dose triple therapy with valsartan, showed no significant pharmacokinetic interactions and exhibited favorable safety and tolerability profiles. []
- HCTZ + Losartan Potassium: This combination proved effective and well-tolerated in hypertensive patients, with a smooth 24-hour blood pressure control. []
Q13: Are there any concerns about this compound causing electrolyte imbalances?
A13: One notable concern with this compound is its potential to cause hypokalemia (low potassium levels), especially with higher doses or prolonged use. [, , ] This is primarily due to increased potassium excretion through the kidneys.
Q14: What is the impact of this compound on serum potassium levels?
A14: Studies highlight the significant impact of HCTZ on serum potassium, with a high prevalence of hypokalemia observed in hypertensive patients treated with this drug. This emphasizes the importance of potassium level monitoring during therapy. []
Q15: Does combining this compound with other drugs affect its potassium-lowering effect?
A15: Combining HCTZ with certain medications can influence its effects on potassium levels:
- Enalapril: Co-administration of enalapril with HCTZ appears to offer a protective effect against hypokalemia, potentially mitigating potassium loss induced by HCTZ. [, ]
- Amiloride: Similarly, the addition of amiloride to HCTZ therapy can help prevent severe hypokalemia and alkalosis that might arise from using HCTZ alone. [, ]
Q16: Are there any ongoing large-scale studies comparing the cardiovascular outcomes of chlorthalidone and this compound?
A17: Yes, the Diuretic Comparison Project (VA Cooperative Study 597) is a large randomized trial aiming to directly compare the effects of chlorthalidone and HCTZ on cardiovascular outcomes in older patients with hypertension. [] This landmark study promises valuable insights into the long-term efficacy and safety of these commonly prescribed diuretics.
Q17: What are the potential advantages of fixed-dose combination therapies incorporating this compound?
A17: Fixed-dose combinations like those containing HCTZ with amlodipine, valsartan, or losartan potassium present several advantages:
- Improved Patient Compliance: Single-pill combinations can enhance medication adherence by simplifying dosing regimens. []
- Synergistic Effects: Combining drugs with different mechanisms of action can lead to enhanced blood pressure control and potentially lower the required doses of individual components. [, ]
- Cost-Effectiveness: Fixed-dose combinations might offer economic benefits compared to using multiple separate medications. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![N-Hydroxy-3-[6-oxo-1-(3-phenylpropyl)-2,3-dihydropyridin-5-yl]propanamide](/img/structure/B1673365.png)
![(2R)-2-[[1-[[(3S)-1-(carboxymethyl)-2-oxo-4,5-dihydro-3H-1-benzazepin-3-yl]carbamoyl]cyclopentyl]methyl]-4-phenylbutanoic acid](/img/structure/B1673368.png)
![6-(7-Methoxy-2-trifluoromethylpyrazolo[1,5-a]pyridin-4-yl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one](/img/structure/B1673371.png)
![3-[(4-Pyridin-2-yl-1,3-thiazol-2-yl)amino]phenol](/img/structure/B1673373.png)
![2-[[2-[[4-ethoxy-3-(5-methyl-4-oxo-7-propyl-1H-imidazo[5,1-f][1,2,4]triazin-2-yl)phenyl]sulfonyl-methylamino]acetyl]-methylamino]-N,N-dimethylacetamide](/img/structure/B1673376.png)