Mifépristone
Vue d'ensemble
Description
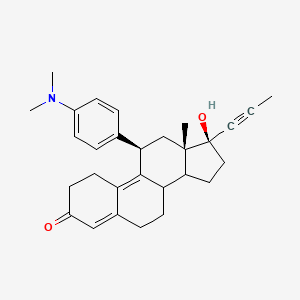
La mifépristone, également connue sous son nom de code de développement RU-486, est un composé stéroïde synthétique de formule chimique C29H35NO2 . Elle est principalement connue pour son utilisation dans les avortements médicaux et la prise en charge des fausses couches précoces. La this compound agit en bloquant l’hormone progestérone, essentielle à la poursuite de la grossesse .
Mécanisme D'action
La mifépristone agit comme un antagoniste des récepteurs de la progestérone et des glucocorticoïdes. En se liant au récepteur de la progestérone, elle empêche l’hormone d’exercer ses effets, ce qui entraîne la dégradation de la muqueuse utérine et l’arrêt de la grossesse. À des doses plus élevées, la this compound bloque également le récepteur des glucocorticoïdes, ce qui est utile pour traiter des affections comme le syndrome de Cushing .
Applications De Recherche Scientifique
Mifepristone has a wide range of scientific research applications, including:
Chemistry: Used as a model compound to study steroidal synthesis and reactions.
Biology: Investigated for its effects on hormone receptors and cellular pathways.
Industry: Utilized in the development of new pharmaceutical formulations and drug delivery systems.
Analyse Biochimique
Biochemical Properties
Mifepristone acts as a competitive progesterone receptor antagonist . In the absence of progesterone, mifepristone acts as a partial agonist . It works by blocking the effects of progesterone, making both the cervix and uterine vessels dilate and causing uterine contraction . Mifepristone is also a glucocorticoid receptor antagonist .
Cellular Effects
Mifepristone has been shown to inhibit ovarian cancer cell proliferation in a dose- and time-dependent manner . It also induced fewer alveoli, enlarged alveolar lumina, and altered the levels of hormones such as estrogen, progesterone, prolactin, growth hormone, corticosterone, and oxytocin, as well as the mRNA expression of these hormonal receptors during pregnancy or early lactation .
Molecular Mechanism
Mifepristone is a selective antagonist of the progesterone receptor at low doses and blocks the glucocorticoid receptor (GR-II) at higher doses . It works by blocking the effects of progesterone, which is necessary for a pregnancy to continue . Mifepristone’s inhibition of progesterone induces bleeding during the luteal phase and in early pregnancy by releasing endogenous prostaglandins from the endometrium or decidua .
Temporal Effects in Laboratory Settings
In a 24-week multicenter, open-label trial, mifepristone produced significant clinical and metabolic improvement in patients with Cushing’s syndrome with an acceptable risk-benefit profile during 6 months of treatment . Mifepristone treatment reduced cellular proliferation and viability of all UM cell lines studied in a concentration-dependent manner .
Dosage Effects in Animal Models
In animal models, mifepristone administration at the dose of 1.20 mg/kg body weight on pregnancy day 4 caused a significant reduction in milk production on lactation day 1, lactation day 2, and lactation day 3 . Mifepristone also induced an increase in the weight of epididymal, perirenal, and gluteofemoral adipose tissues .
Metabolic Pathways
Mifepristone is extensively metabolised by demethylation and hydroxylation, the initial metabolic steps are catalysed by the cytochrome P450 (CYP) enzyme CYP3A4 . The three most proximal metabolites, namely the monodemethylated, didemethylated, and hydroxylated metabolites of mifepristone, all retain considerable affinity toward the human progesterone and glucocorticoid receptors .
Transport and Distribution
The serum transport protein α1-acid glycoprotein (AAG) regulates the serum kinetics of mifepristone . Binding to AAG limits the tissue availability of mifepristone, explaining the low metabolic clearance rate of 0.55 L/kg/day and the low volume of distribution of mifepristone .
Subcellular Localization
Mifepristone markedly reduces cdk2 activity likely due to increased association of cdk2 with the cdk inhibitors p21 cip1 and p27 kip1 and reduced nuclear cdk2/cyclin E complex availability . This suggests that mifepristone may regulate mitochondrial function through the control of mitochondrial gene expression .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La mifépristone est synthétisée par un processus en plusieurs étapes impliquant plusieurs intermédiaires clés. La synthèse commence par la préparation de la 11β-[4-(diméthylamino)phényl]-17β-hydroxy-17α-(1-propynyl)estra-4,9-dièn-3-one. Cet intermédiaire est ensuite soumis à diverses réactions chimiques, y compris l’hydroxylation et l’alkylation, pour produire le composé final .
Méthodes de production industrielle : Dans les milieux industriels, la this compound est produite en utilisant une méthode de granulation humide. Cela implique de mélanger le principe actif pharmaceutique avec des excipients tels que l’amidon et la cellulose microcristalline, suivis d’une granulation avec un mélange eau/alcool. Les granules sont ensuite séchées et comprimées en comprimés .
Analyse Des Réactions Chimiques
Types de réactions : La mifépristone subit plusieurs types de réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former divers métabolites.
Réduction : Les réactions de réduction peuvent modifier le groupe cétone de la this compound.
Substitution : Des réactions de substitution peuvent se produire au niveau du groupe diméthylamino.
Réactifs et Conditions Courants :
Oxydation : Les agents oxydants courants comprennent le permanganate de potassium et le trioxyde de chrome.
Réduction : Des agents réducteurs tels que le borohydrure de sodium et l’hydrure de lithium et d’aluminium sont utilisés.
Substitution : Des réactifs comme les halogénoalcanes et les amines sont utilisés pour les réactions de substitution.
Principaux produits formés : Les principaux produits formés à partir de ces réactions comprennent divers métabolites hydroxylés et déméthylés, tels que la N-desméthyl-mifépristone et la 22-hydroxy-mifépristone .
4. Applications de la Recherche Scientifique
La this compound a un large éventail d’applications de recherche scientifique, notamment :
Chimie : Utilisée comme composé modèle pour étudier la synthèse et les réactions stéroïdiennes.
Biologie : Étudiée pour ses effets sur les récepteurs hormonaux et les voies cellulaires.
Industrie : Utilisée dans le développement de nouvelles formulations pharmaceutiques et systèmes d’administration de médicaments.
Comparaison Avec Des Composés Similaires
La mifépristone appartient à une classe de composés appelés antagonistes des récepteurs de la progestérone. Les composés similaires comprennent :
Levonorgestrel : Utilisé pour la contraception d’urgence, mais agit principalement comme un progestatif plutôt qu’un antagoniste.
Acétate d’ulipristal : Un autre modulateur des récepteurs de la progestérone utilisé pour la contraception d’urgence et le traitement des fibromes.
Unicité de la this compound : La capacité unique de la this compound à agir à la fois comme un antagoniste des récepteurs de la progestérone et des glucocorticoïdes la distingue des autres composés de sa classe. Cette double action la rend très efficace pour une gamme d’applications médicales, allant de l’arrêt des grossesses à la prise en charge des troubles hormonaux .
Propriétés
IUPAC Name |
(8S,11R,13S,14S,17S)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VKHAHZOOUSRJNA-GCNJZUOMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC#CC1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC#C[C@@]1(CC[C@@H]2[C@@]1(C[C@@H](C3=C4CCC(=O)C=C4CC[C@@H]23)C5=CC=C(C=C5)N(C)C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H35NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5023322 | |
| Record name | Mifepristone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023322 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
429.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
7 [ug/mL] (The mean of the results at pH 7.4), Poorly soluble, Very soluble in methanol, chloroform, and acetone and poorly soluble in water, hexane, and isopropyl ether., In water, 5.0X10-2 mg/L at 25 °C /Estimated/, 3.36e-03 g/L | |
| Record name | SID11533034 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
8.0X10-14 mm Hg at 25 °C /Estimated/ | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The anti-progestational activity of mifepristone results from competitive interaction with progesterone at progesterone-receptor sites. Based on studies with various oral doses in several animal species (mouse, rat, rabbit and monkey), the compound inhibits the activity of endogenous or exogenous progesterone. The termination of pregnancy results. In the treatment of Cushing's syndrome, Mifepristone blocks the binding of cortisol to its receptor. It does not decrease cortisol production but reduces the effects of excess cortisol, such as high blood sugar levels., Mifepristone competitively inhibits the actions of progesterone at progesterone-receptor sites, resulting in termination of pregnancy.The combination of mifepristone and misoprostol causes expulsion of the products of conception through decidual necrosis, myometrial contractions, and cervical softening., When administered in the early stages of pregnancy, mifepristone causes decidual breakdown by blockade of uterine progesterone receptors. This leads to detachment of the blastocyte, which decreases hCG production. This in turn causes a decrease in progesterone secretion from the corpus luteum, which further accentuates decidual breakdown. Decreased endogenous progesterone coupled with blockade of progesterone receptors in the uterus increases prostaglandin levels and sensitizes the myometrium to the contractile actions of prostaglandins., In addition, mifepristone promotes uterine contractions and softening of the cervix and sensitizes the myometrium to effects of prostaglandins (e.g., misoprostol) that stimulate uterine contraction and expulsion of the products of conception. In the absence of progesterone, mifepristone acts as a partial progestin agonist. At dosages higher than those used for termination of pregnancy, mifepristone also exhibits antiglucocorticoid activity. The drug also has been shown to have weak antiandrogenic activity. | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow powder | |
CAS No. |
84371-65-3 | |
| Record name | Mifepristone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=84371-65-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mifepristone [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0084371653 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mifepristone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023322 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Estra-4,9-dien-3-one, 11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propyn-1-yl)-, (11β,17β) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.911 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MIFEPRISTONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/320T6RNW1F | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
191-196 °C, 150 °C, 191 - 196 °C | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![N-cyclopropyl-6-[(6,7-dimethoxyquinolin-4-yl)oxy]naphthalene-1-carboxamide](/img/structure/B1683809.png)