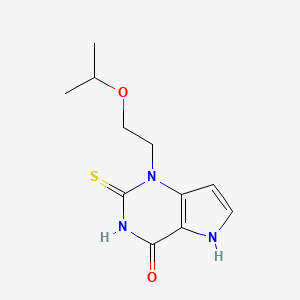
ミフェプリストン
概要
説明
ミフェプリストンは、開発コード名 RU-486 で知られる合成ステロイド化合物であり、化学式は C29H35NO2 です。主に、薬物による妊娠中絶と初期流産の管理に使用されています。 ミフェプリストンは、妊娠の継続に不可欠なホルモンであるプロゲステロンを阻害することにより作用します .
2. 製法
合成経路と反応条件: ミフェプリストンは、いくつかの重要な中間体を用いた複数段階のプロセスによって合成されます。合成は、11β-[4-(ジメチルアミノ)フェニル]-17β-ヒドロキシ-17α-(1-プロピニル)エストラ-4,9-ジエン-3-オンの調製から始まります。 この中間体は、その後、ヒドロキシル化とアルキル化を含む様々な化学反応にかけられ、最終的な化合物が生成されます .
工業的製造方法: 工業的には、ミフェプリストンは湿式造粒法を用いて製造されます。これは、薬学的に活性な成分をデンプンや微結晶セルロースなどの賦形剤と混合し、水/アルコール混合物で造粒する工程です。 その後、顆粒を乾燥させ、錠剤に圧縮します .
3. 化学反応解析
反応の種類: ミフェプリストンは、以下の様な様々な化学反応を起こします。
酸化: ミフェプリストンは酸化されて、様々な代謝産物を生成することができます。
還元: 還元反応は、ミフェプリストン中のケトン基を修飾することができます。
一般的な試薬と条件:
酸化: 一般的な酸化剤としては、過マンガン酸カリウムや三酸化クロムがあります。
還元: 水素化ホウ素ナトリウムや水素化アルミニウムリチウムなどの還元剤が使用されます。
生成される主な生成物: これらの反応から生成される主な生成物には、N-デスメチルミフェプリストンや22-ヒドロキシミフェプリストンなどの様々なヒドロキシル化および脱メチル化された代謝産物が含まれます .
4. 科学研究への応用
ミフェプリストンは、以下の様な幅広い科学研究分野で応用されています。
化学: ステロイド合成と反応を研究するためのモデル化合物として使用されます。
生物学: ホルモン受容体や細胞経路への影響について調査されています。
作用機序
ミフェプリストンは、プロゲステロン受容体とグルココルチコイド受容体の両方のアンタゴニストとして作用します。プロゲステロン受容体に結合することで、ホルモンがその効果を発揮することを阻止し、子宮内膜の分解と妊娠の中絶を引き起こします。 高用量では、ミフェプリストンはグルココルチコイド受容体もブロックし、クッシング症候群などの治療に役立ちます .
科学的研究の応用
Mifepristone has a wide range of scientific research applications, including:
Chemistry: Used as a model compound to study steroidal synthesis and reactions.
Biology: Investigated for its effects on hormone receptors and cellular pathways.
Industry: Utilized in the development of new pharmaceutical formulations and drug delivery systems.
生化学分析
Biochemical Properties
Mifepristone acts as a competitive progesterone receptor antagonist . In the absence of progesterone, mifepristone acts as a partial agonist . It works by blocking the effects of progesterone, making both the cervix and uterine vessels dilate and causing uterine contraction . Mifepristone is also a glucocorticoid receptor antagonist .
Cellular Effects
Mifepristone has been shown to inhibit ovarian cancer cell proliferation in a dose- and time-dependent manner . It also induced fewer alveoli, enlarged alveolar lumina, and altered the levels of hormones such as estrogen, progesterone, prolactin, growth hormone, corticosterone, and oxytocin, as well as the mRNA expression of these hormonal receptors during pregnancy or early lactation .
Molecular Mechanism
Mifepristone is a selective antagonist of the progesterone receptor at low doses and blocks the glucocorticoid receptor (GR-II) at higher doses . It works by blocking the effects of progesterone, which is necessary for a pregnancy to continue . Mifepristone’s inhibition of progesterone induces bleeding during the luteal phase and in early pregnancy by releasing endogenous prostaglandins from the endometrium or decidua .
Temporal Effects in Laboratory Settings
In a 24-week multicenter, open-label trial, mifepristone produced significant clinical and metabolic improvement in patients with Cushing’s syndrome with an acceptable risk-benefit profile during 6 months of treatment . Mifepristone treatment reduced cellular proliferation and viability of all UM cell lines studied in a concentration-dependent manner .
Dosage Effects in Animal Models
In animal models, mifepristone administration at the dose of 1.20 mg/kg body weight on pregnancy day 4 caused a significant reduction in milk production on lactation day 1, lactation day 2, and lactation day 3 . Mifepristone also induced an increase in the weight of epididymal, perirenal, and gluteofemoral adipose tissues .
Metabolic Pathways
Mifepristone is extensively metabolised by demethylation and hydroxylation, the initial metabolic steps are catalysed by the cytochrome P450 (CYP) enzyme CYP3A4 . The three most proximal metabolites, namely the monodemethylated, didemethylated, and hydroxylated metabolites of mifepristone, all retain considerable affinity toward the human progesterone and glucocorticoid receptors .
Transport and Distribution
The serum transport protein α1-acid glycoprotein (AAG) regulates the serum kinetics of mifepristone . Binding to AAG limits the tissue availability of mifepristone, explaining the low metabolic clearance rate of 0.55 L/kg/day and the low volume of distribution of mifepristone .
Subcellular Localization
Mifepristone markedly reduces cdk2 activity likely due to increased association of cdk2 with the cdk inhibitors p21 cip1 and p27 kip1 and reduced nuclear cdk2/cyclin E complex availability . This suggests that mifepristone may regulate mitochondrial function through the control of mitochondrial gene expression .
準備方法
Synthetic Routes and Reaction Conditions: Mifepristone is synthesized through a multi-step process involving several key intermediates. The synthesis begins with the preparation of 11β-[4-(dimethylamino)phenyl]-17β-hydroxy-17α-(1-propynyl)estra-4,9-dien-3-one. This intermediate is then subjected to various chemical reactions, including hydroxylation and alkylation, to produce the final compound .
Industrial Production Methods: In industrial settings, mifepristone is produced using a wet granulation method. This involves mixing the active pharmaceutical ingredient with excipients such as starch and microcrystalline cellulose, followed by granulation with a water/alcohol mixture. The granules are then dried and compressed into tablets .
化学反応の分析
Types of Reactions: Mifepristone undergoes several types of chemical reactions, including:
Oxidation: Mifepristone can be oxidized to form various metabolites.
Reduction: Reduction reactions can modify the ketone group in mifepristone.
Substitution: Substitution reactions can occur at the dimethylamino group.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and chromium trioxide.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Reagents like alkyl halides and amines are employed for substitution reactions.
Major Products Formed: The major products formed from these reactions include various hydroxylated and demethylated metabolites, such as N-desmethyl-mifepristone and 22-hydroxy-mifepristone .
類似化合物との比較
ミフェプリストンは、プロゲステロン受容体アンタゴニストとして知られる化合物群に属します。類似の化合物には、以下のようなものがあります。
レボノルゲストレル: 緊急避妊に使用されますが、アンタゴニストではなく、主にプロゲステロンとして作用します.
ユリプリスタルアセテート: 緊急避妊と子宮筋腫の治療に使用される、別のプロゲステロン受容体モジュレーターです.
ミフェプリストンの独自性: ミフェプリストンは、プロゲステロンとグルココルチコイド受容体の両方のアンタゴニストとして作用するという独自の特徴を持つため、このクラスの他の化合物とは一線を画しています。 この二重の作用により、妊娠の終了からホルモン障害の管理まで、幅広い医療用途で非常に効果的です .
特性
IUPAC Name |
(8S,11R,13S,14S,17S)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VKHAHZOOUSRJNA-GCNJZUOMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC#CC1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC#C[C@@]1(CC[C@@H]2[C@@]1(C[C@@H](C3=C4CCC(=O)C=C4CC[C@@H]23)C5=CC=C(C=C5)N(C)C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H35NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5023322 | |
| Record name | Mifepristone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023322 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
429.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
7 [ug/mL] (The mean of the results at pH 7.4), Poorly soluble, Very soluble in methanol, chloroform, and acetone and poorly soluble in water, hexane, and isopropyl ether., In water, 5.0X10-2 mg/L at 25 °C /Estimated/, 3.36e-03 g/L | |
| Record name | SID11533034 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
8.0X10-14 mm Hg at 25 °C /Estimated/ | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The anti-progestational activity of mifepristone results from competitive interaction with progesterone at progesterone-receptor sites. Based on studies with various oral doses in several animal species (mouse, rat, rabbit and monkey), the compound inhibits the activity of endogenous or exogenous progesterone. The termination of pregnancy results. In the treatment of Cushing's syndrome, Mifepristone blocks the binding of cortisol to its receptor. It does not decrease cortisol production but reduces the effects of excess cortisol, such as high blood sugar levels., Mifepristone competitively inhibits the actions of progesterone at progesterone-receptor sites, resulting in termination of pregnancy.The combination of mifepristone and misoprostol causes expulsion of the products of conception through decidual necrosis, myometrial contractions, and cervical softening., When administered in the early stages of pregnancy, mifepristone causes decidual breakdown by blockade of uterine progesterone receptors. This leads to detachment of the blastocyte, which decreases hCG production. This in turn causes a decrease in progesterone secretion from the corpus luteum, which further accentuates decidual breakdown. Decreased endogenous progesterone coupled with blockade of progesterone receptors in the uterus increases prostaglandin levels and sensitizes the myometrium to the contractile actions of prostaglandins., In addition, mifepristone promotes uterine contractions and softening of the cervix and sensitizes the myometrium to effects of prostaglandins (e.g., misoprostol) that stimulate uterine contraction and expulsion of the products of conception. In the absence of progesterone, mifepristone acts as a partial progestin agonist. At dosages higher than those used for termination of pregnancy, mifepristone also exhibits antiglucocorticoid activity. The drug also has been shown to have weak antiandrogenic activity. | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow powder | |
CAS No. |
84371-65-3 | |
| Record name | Mifepristone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=84371-65-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mifepristone [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0084371653 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mifepristone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023322 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Estra-4,9-dien-3-one, 11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propyn-1-yl)-, (11β,17β) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.911 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MIFEPRISTONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/320T6RNW1F | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
191-196 °C, 150 °C, 191 - 196 °C | |
| Record name | Mifepristone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00834 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MIFEPRISTONE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mifepristone | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014972 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![N-cyclopropyl-6-[(6,7-dimethoxyquinolin-4-yl)oxy]naphthalene-1-carboxamide](/img/structure/B1683809.png)