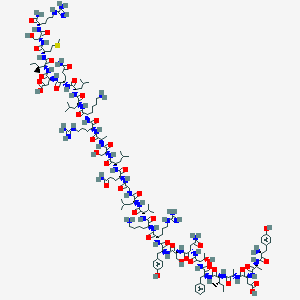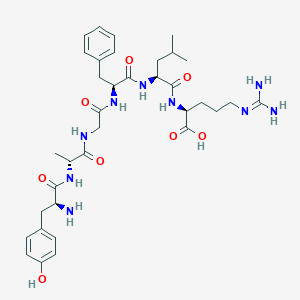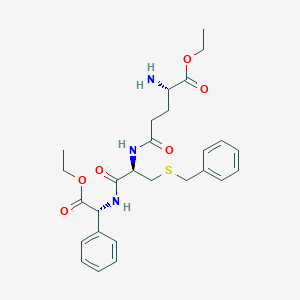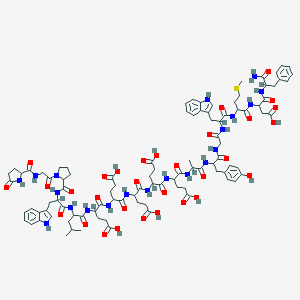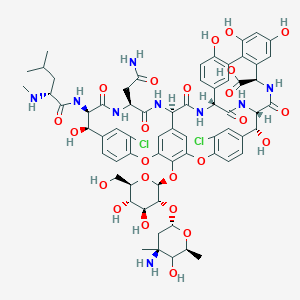
Vancomycine
Vue d'ensemble
Description
La vancomycine est un antibiotique glycopeptidique principalement utilisé pour traiter les infections bactériennes graves causées par des bactéries à Gram positif, notamment le Staphylococcus aureus résistant à la méthicilline (SARM) et Clostridium difficile . Elle a été isolée pour la première fois à partir d'un échantillon de sol à Bornéo en 1956 et est depuis devenue un outil essentiel dans la lutte contre les infections résistantes aux antibiotiques .
Mécanisme D'action
Target of Action
Vancomycin, a glycopeptide antibiotic, primarily targets the D-alanyl-D-alanine (D-Ala-D-Ala) terminus of cell wall precursor units in bacteria . This terminal sequence plays a crucial role in bacterial cell wall synthesis . The antibiotic is particularly effective against severe bacterial infections such as MRSA (methicillin-resistant Staphylococcus aureus) infections .
Mode of Action
Vancomycin operates by inhibiting cell wall synthesis in bacteria . It binds to the D-Ala-D-Ala terminus of cell wall precursor units, preventing their incorporation into the cell wall . This action weakens the bacterial cell wall, leading to cell lysis and death . There is also evidence that vancomycin alters the permeability of the cell membrane and selectively inhibits ribonucleic acid synthesis .
Biochemical Pathways
The primary biochemical pathway affected by vancomycin is the synthesis of the bacterial cell wall. By binding to the D-Ala-D-Ala terminus of cell wall precursor units, vancomycin prevents the transglycosylation step in peptidoglycan polymerization . This action disrupts the formation of the bacterial cell wall, leading to cell lysis and death .
Pharmacokinetics
In patients with normal creatinine clearance, vancomycin has an α-distribution phase of ∼30 min to 1 hour and a β-elimination half-life of 6–12 hours. The volume of distribution is 0.4–1 L/kg . The binding of vancomycin to protein has been reported to range from 10% to 50% .
Result of Action
The molecular effect of vancomycin’s action is the disruption of the bacterial cell wall, which leads to cell lysis and death . On a cellular level, vancomycin causes changes in the permeability of the cell membrane and selectively inhibits ribonucleic acid synthesis . This results in the inhibition of bacterial growth and proliferation.
Action Environment
Environmental factors can influence the action, efficacy, and stability of vancomycin. For instance, regional variations and climate types (temperate and monsoon climates) have been found to significantly affect the resistance rate of vancomycin-resistant Enterococcus faecalis . Furthermore, the physical environment in healthcare settings, such as the cleanliness of surfaces, can impact the transmission of vancomycin-resistant bacteria .
Applications De Recherche Scientifique
Vancomycin has a wide range of applications in scientific research:
Chemistry: Used as a standard in analytical methods to study antibiotic properties and interactions.
Biology: Essential in studying bacterial cell wall synthesis and resistance mechanisms.
Medicine: Critical in treating severe infections, especially those resistant to other antibiotics.
Industry: Used in the development of new antibiotics and in quality control processes.
Analyse Biochimique
Biochemical Properties
Vancomycin acts by binding to the D-alanyl-D-alanine moieties of the NAM/NAG-peptides, preventing the incorporation of these subunits into the peptidoglycan matrix, which forms the major structural component of Gram-positive bacterial cell walls . This interaction is non-covalent and results in the inhibition of cell wall synthesis . Vancomycin’s activity is concentration-independent, also referred to as “time-dependent,” and its clinical activity can be affected by factors such as variable tissue distribution, inoculum size, and emerging resistance .
Cellular Effects
Vancomycin primarily targets bacterial cells, where it disrupts cell wall synthesis, leading to cell death . It has been shown to have variable effects on different types of cells. For example, it has been found to reduce cell viability by 20% to 30% with increasing concentration up from 0.2 mg/mL . It also induces oxidative stress, as indicated by a reduction in the antioxidant activity of SOD2 and an increase in the MDA level .
Molecular Mechanism
Vancomycin exerts its effects at the molecular level by forming an intricate network of hydrogen bonds with the D-Ala-D-Ala region of Lipid II, interfering with the peptidoglycan layer maturation process . This binding prevents the incorporation of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG)-peptide subunits into the peptidoglycan matrix .
Temporal Effects in Laboratory Settings
The pharmacokinetic profile of vancomycin is complex and can be characterized by either a 2- or 3-compartment pharmacokinetic profile . In patients with normal creatinine clearance, vancomycin has an α-distribution phase of approximately 30 minutes to 1 hour and a β-elimination half-life of 6–12 hours . Over time, the effects of vancomycin can change due to factors such as the development of resistance .
Dosage Effects in Animal Models
In animal models, the effects of vancomycin can vary with different dosages. For instance, in beagle dogs, vancomycin is administered intravenously at a dose rate of 20 mg/kg over a 1-hour period at 12-hour intervals . The effects of vancomycin in animal models can also be influenced by factors such as the animal’s health status and the presence of other medications.
Metabolic Pathways
Vancomycin is not appreciably metabolized and is eliminated primarily via the renal route, with more than 80% to 90% recovered unchanged in urine within 24 hours after administration of a single dose . It has been suggested that vancomycin disrupts amino acids metabolism, fatty acids biosynthesis, energy metabolism, and lipid metabolism .
Transport and Distribution
Vancomycin is administered intravenously and has a volume of distribution of 0.4–1 L/kg . It penetrates into most body spaces, although the concentrations obtained are variable and somewhat dependent on the degree of inflammation present .
Subcellular Localization
Vancomycin accumulates in lysosomes of proximal tubular cells throughout 10 days of treatment . The subcellular localization of vancomycin in the renal cortices of rats was determined with ultrathin sections by immunogold labeling .
Méthodes De Préparation
La vancomycine est produite par fermentation de la bactérie Amycolatopsis orientalis . La production industrielle implique plusieurs étapes :
Fermentation : Amycolatopsis orientalis est cultivée dans un milieu riche en nutriments pour produire de la this compound.
Extraction : L'antibiotique est extrait du bouillon de fermentation à l'aide de solvants organiques.
Purification : La chromatographie liquide haute performance (HPLC) est couramment utilisée pour purifier la this compound.
Analyse Des Réactions Chimiques
La vancomycine subit diverses réactions chimiques, notamment :
Oxydation et réduction : Ces réactions peuvent modifier les groupes fonctionnels sur la molécule de this compound, modifiant potentiellement son activité.
Réactions de substitution : Les réactifs courants comprennent l'acétonitrile et le méthanol, utilisés dans les méthodes chromatographiques.
Principaux produits : Le produit principal est la this compound purifiée, qui conserve ses propriétés antibiotiques.
4. Applications de la recherche scientifique
La this compound a un large éventail d'applications dans la recherche scientifique :
Biologie : Essentiel pour étudier la synthèse de la paroi cellulaire bactérienne et les mécanismes de résistance.
Industrie : Utilisé dans le développement de nouveaux antibiotiques et dans les processus de contrôle qualité.
5. Mécanisme d'action
La this compound agit en inhibant la synthèse de la paroi cellulaire chez les bactéries. Elle se lie à la terminaison D-alanyl-D-alanine des unités précurseurs de la paroi cellulaire, empêchant leur incorporation dans la paroi cellulaire et affaiblissant ainsi la paroi cellulaire bactérienne, ce qui conduit à la lyse cellulaire et à la mort . Ce mécanisme le rend très efficace contre les bactéries à Gram positif .
Comparaison Avec Des Composés Similaires
La vancomycine est souvent comparée à d'autres antibiotiques, tels que :
Linézolide : Une oxazolidinone synthétique qui inhibe la synthèse protéique.
Daptomycine : Un lipopeptide qui perturbe la fonction de la membrane cellulaire.
Tigecycline : Une glycylcycline qui inhibe la synthèse protéique.
Teicoplanine : Un autre glycopeptide ayant un mécanisme d'action similaire.
L'unicité de la this compound réside dans sa capacité à traiter les infections graves causées par des bactéries résistantes aux antibiotiques, ce qui en fait un outil vital en médecine moderne .
Propriétés
Numéro CAS |
1404-90-6 |
|---|---|
Formule moléculaire |
C66H75Cl2N9O24 |
Poids moléculaire |
1449.2 g/mol |
Nom IUPAC |
48-[3-[(4S)-4-amino-5-hydroxy-4,6-dimethyloxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-22-(2-amino-2-oxoethyl)-5,15-dichloro-2,18,32,35,37-pentahydroxy-19-[[4-methyl-2-(methylamino)pentanoyl]amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta-3,5,8(48),9,11,14,16,29(45),30,32,34(39),35,37,46,49-pentadecaene-40-carboxylic acid |
InChI |
InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24?,34?,35?,42?,44?,46?,47?,48?,49?,50?,51?,52?,53?,54?,56?,57?,65?,66-/m0/s1 |
Clé InChI |
MYPYJXKWCTUITO-BSRCFTEOSA-N |
SMILES |
CC1C(C(CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(C(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
SMILES isomérique |
CC1C([C@@](CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(C(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
SMILES canonique |
CC1C(C(CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(C(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
Apparence |
White to off-white solid powder |
| Vancomycin is an antibiotic used to treat a number of bacterial infections. It is a member of the glycopeptide antibiotic class and is effective mostly against Gram-positive bacteria. | |
Point d'ébullition |
N/A |
melting_point |
N/A |
| 1404-90-6 | |
Description physique |
Tan to brown solid; [HSDB] |
Pictogrammes |
Health Hazard |
Pureté |
>98% (or refer to the Certificate of Analysis) |
Numéros CAS associés |
1404-93-9 (hydrochloride) 64685-75-2 (sulfate) |
Durée de conservation |
When reconstituted with sterile water for injection, vancomycin hydrochloride injection is stable for 2 weeks at room temperature; the manufacturers state that reconstituted injections may be stored for 96 hours at 2 - 8 °C without substantial loss of potency. When reconstituted as directed in 0.9% sodium chloride injection or 5% dextrose injection, solutions prepared from ADD-Vantage vials of the drug are stable for 24 hours at room temperature. Vancomycin solutions containing 5 mg/mL in 0.9% sodium chloride injection or 5% dextrose injection are reportedly stable for at least 17 days when stored at 24 °C in glass or PVC containers and for at least 63 days when stored at 5 °C or -10 °C in glass containers. Following reconstitution with sterile water for injection as directed, vancomycin solutions that have been further diluted to a concentration of 5 mg/mL in 5 - 30% dextrose injection are stable when stored in plastic syringes for 24 hours at 4 eg C and then subsequently for 2 hours at room temperature. Solutions are stable for two weeks at room temp or longer if refrigerated. /Hydrochloride/ |
Solubilité |
White solid; solubility in water: greater than 100 mg/mL; moderately soluble in methanol; insoluble in higher alcohols, acetone, ether; UV max absorption (water): 282 nm (e = 40, 1%, 1 cm) /Vancomycin hydrochloride/ |
Source |
Synthetic |
Synonymes |
AB-Vancomycin Diatracin Hydrochloride, Vancomycin Sulfate, Vancomycin Vanco Azupharma VANCO-cell Vanco-saar Vancocin Vancocin HCl Vancocine Vancomicina Abbott Vancomicina Chiesi Vancomicina Combino Phar Vancomicina Norman Vancomycin Vancomycin Hexal Vancomycin Hydrochloride Vancomycin Lilly Vancomycin Phosphate (1:2) Vancomycin Phosphate (1:2), Decahydrate Vancomycin Sulfate Vancomycin-ratiopharm Vancomycine Dakota |
Origine du produit |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.