Raltegravir
Vue d'ensemble
Description
Le Raltegravir est un médicament antirétroviral utilisé en association avec d'autres médicaments pour traiter l'infection par le virus de l'immunodéficience humaine (VIH). C'est le premier inhibiteur de la transférence de brin de l'intégrase du VIH approuvé, qui bloque le fonctionnement de l'intégrase du VIH, une enzyme nécessaire à la réplication virale . Le this compound a été approuvé pour un usage médical aux États-Unis en 2007 et figure sur la Liste des médicaments essentiels de l'Organisation mondiale de la santé .
Mécanisme D'action
Target of Action
Raltegravir primarily targets the HIV-1 integrase enzyme . This enzyme plays a crucial role in the life cycle of the HIV-1 virus, as it is responsible for the integration of the viral DNA into the host cell’s chromosomes .
Mode of Action
This compound acts as an integrase inhibitor . It binds to the integrase enzyme and inhibits the strand transfer step of HIV-1 integration . This prevents the viral genome from being incorporated into the human genome, thereby halting the replication of the virus .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the HIV-1 integration process . This process involves three essential steps: formation of the preintegration viral DNA complex, 3′ processing, and strand transfer . This compound interferes with the final step, strand transfer, thereby preventing the integration of the viral DNA into the host cell’s chromosomes .
Pharmacokinetics
This compound is absorbed from the gastrointestinal tract . It is approximately 83% bound to human plasma protein and is minimally distributed into red blood cells . The major mechanism of clearance of this compound in humans is glucuronidation mediated by UGT1A1 , with renal clearance of unchanged drug being a minor pathway of elimination .
Result of Action
The inhibition of the HIV-1 integrase enzyme by this compound results in the prevention of the viral genome from being incorporated into the human genome . This effectively halts the replication of the virus, thereby reducing the viral load in the body .
Action Environment
This compound’s action, efficacy, and stability can be influenced by various environmental factors. For instance, co-administered medications that affect gastric pH can impact the absorption of this compound . Additionally, genetic variations in UGT isoenzymes, which are involved in the metabolism of this compound, may affect its exposure .
Applications De Recherche Scientifique
Analyse Biochimique
Biochemical Properties
Raltegravir plays a significant role in biochemical reactions as it inhibits HIV integrase, a key enzyme in the HIV life cycle . This enzyme is responsible for the integration of the HIV-1 viral DNA, generated by reverse transcription of the RNA genome, into the host cell chromosomes . By inhibiting this process, this compound prevents the replication of the virus .
Cellular Effects
This compound has a profound impact on various types of cells infected with HIV-1. By inhibiting the integrase enzyme, it prevents the integration of the viral DNA into the host cell chromosomes . This halts the production of new viruses, thereby influencing cell function and impacting cellular processes such as cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of the HIV integrase enzyme to prevent the viral genome from being incorporated into the human genome . This compound is primarily metabolized by glucuronidation .
Temporal Effects in Laboratory Settings
In laboratory settings, this compound has shown potent antiretroviral activity and is well tolerated in HIV-1–infected individuals . Specific resistance mutations have been identified in patients failing to respond to treatment with this compound
Dosage Effects in Animal Models
In animal models, the effects of this compound have been observed to vary with different dosages . More detailed studies are required to understand the threshold effects and any toxic or adverse effects at high doses.
Metabolic Pathways
This compound is involved in metabolic pathways where it is primarily metabolized by glucuronidation . This metabolic process does not involve the liver, thus reducing the potential for drug-drug interactions .
Transport and Distribution
It is known that this compound is approximately 83% bound to human plasma protein and is minimally distributed into red blood cells .
Subcellular Localization
Given its mechanism of action, it can be inferred that this compound acts in the nucleus of the cell where it prevents the integration of the HIV-1 viral DNA into the host cell chromosomes .
Méthodes De Préparation
Le Raltegravir est synthétisé par un processus en plusieurs étapes impliquant plusieurs intermédiaires clés. L'un des défis importants de sa synthèse est la N-méthylation sélective d'un intermédiaire de pyrimidone. La voie de synthèse implique généralement les étapes suivantes :
N-alkylation : L'intermédiaire de pyrimidone est alkylé en utilisant du (chlorométhyl)diméthylchlorosilane et du fluorure de potassium.
Amidation : L'intermédiaire alkylé subit une amidation avec une amine.
Désilylation : L'étape finale implique une désilylation en utilisant du fluorure de potassium dans le méthanol.
Les méthodes de production industrielle impliquent l'optimisation de ces étapes afin d'obtenir des rendements et une pureté élevés. Le procédé est mis à l'échelle pour produire du raltégravir potassium, qui est la forme pharmaceutiquement active utilisée dans les médicaments .
Analyse Des Réactions Chimiques
Le Raltegravir subit diverses réactions chimiques, notamment :
Oxydation : Le this compound peut être oxydé dans des conditions spécifiques, conduisant à la formation de divers produits d'oxydation.
Réduction : Des réactions de réduction peuvent être effectuées sur le this compound pour modifier ses groupes fonctionnels.
Substitution : Le this compound peut subir des réactions de substitution, en particulier au niveau du groupe fluorobenzyl.
Les réactifs courants utilisés dans ces réactions comprennent les agents oxydants comme le peroxyde d'hydrogène, les agents réducteurs comme le borohydrure de sodium et les nucléophiles pour les réactions de substitution. Les principaux produits formés dépendent des conditions de réaction et des réactifs spécifiques utilisés .
Applications de la recherche scientifique
Le this compound a plusieurs applications de recherche scientifique, notamment :
Médecine : Le this compound est principalement utilisé dans le traitement de l'infection à VIH-1.
Biologie : Le this compound est utilisé dans la recherche pour étudier les mécanismes d'intégration et de réplication du VIH.
Mécanisme d'action
Le this compound inhibe l'activité catalytique de l'intégrase du VIH, empêchant l'intégration du génome viral dans le génome humain. Cette inhibition bloque l'étape de transfert de brin de l'intégration du VIH-1, qui est essentielle à la réplication virale. Le this compound est principalement métabolisé par glucuronidation .
Comparaison Avec Des Composés Similaires
Le Raltegravir est comparé à d'autres inhibiteurs de la transférence de brin de l'intégrase (INSTI) tels que l'elvitégravir, le dolutegravir, le bictegravir et le cabotégravir. Ces composés partagent un mécanisme d'action similaire, mais diffèrent par leurs propriétés pharmacocinétiques et pharmacodynamiques :
Elvitégravir : Nécessite un renforcement avec le cobicistat pour des niveaux plasmatiques efficaces.
Dolutegravir : Connu pour sa forte barrière à la résistance et sa posologie une fois par jour.
Bictegravir : Co-formulé avec l'emtricitabine et le ténofovir alafénamide dans un schéma thérapeutique à comprimé unique.
Cabotégravir : Disponible sous forme de formulation injectable à action prolongée.
Le this compound est unique par ses données de sécurité étendues et son utilisation chez les patients traités pour une infection à VIH résistante aux médicaments .
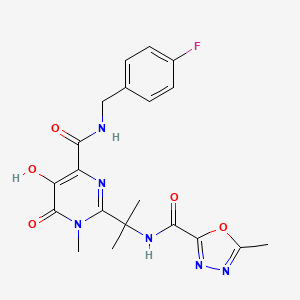
Propriétés
IUPAC Name |
N-[2-[4-[(4-fluorophenyl)methylcarbamoyl]-5-hydroxy-1-methyl-6-oxopyrimidin-2-yl]propan-2-yl]-5-methyl-1,3,4-oxadiazole-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CZFFBEXEKNGXKS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H21FN6O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2048660 | |
| Record name | Raltegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2048660 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
444.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Raltegravir inhibits HIV integrase to prevent the viral genome being incorporated into the human genome. Raltegravir is primarily metabolized by glucuronidation., Raltegravir inhibits the catalytic activity of HIV-1 integrase, an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the covalent insertion, or integration, of unintegrated linear HIV-1 DNA into the host cell genome preventing the formation of the HIV-1 provirus. The provirus is required to direct the production of progeny virus, so inhibiting integration prevents propagation of the viral infection. Raltegravir did not significantly inhibit human phosphoryltransferases including DNA polymerases alpha, beta, and gamma. | |
| Record name | Raltegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06817 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Raltegravir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8124 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
518048-05-0 | |
| Record name | Raltegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=518048-05-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Raltegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0518048050 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Raltegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06817 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Raltegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2048660 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.124.631 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | RALTEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/22VKV8053U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Raltegravir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8124 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Synthesis routes and methods
Procedure details





Q1: How does Raltegravir exert its antiretroviral effect?
A1: this compound is a potent inhibitor of HIV-1 integrase, specifically targeting the strand transfer step of the viral integration process. [] This prevents the integration of the viral genome into the host cell's DNA, a crucial step for viral replication. [, ]
Q2: What is the consequence of inhibiting HIV-1 integrase?
A2: Blocking the strand transfer step by this compound leads to the accumulation of unintegrated viral DNA in the cytoplasm of infected cells. [, ] This ultimately prevents the production of new infectious viral particles, effectively suppressing viral replication. [, , ]
Q3: What is the significance of this compound's target in the HIV life cycle?
A3: HIV-1 integrase is a viral enzyme essential for viral replication and is not found in human cells. [] Targeting this unique enzyme offers a high degree of selectivity, minimizing the potential for adverse effects on host cell processes. []
Q4: What is the molecular formula and weight of this compound?
A4: This information is not provided in the provided scientific papers. Please consult comprehensive chemical databases like PubChem or DrugBank for detailed structural information.
Q5: How stable is this compound under various storage conditions?
A5: While the provided research doesn't specify exact storage conditions, one study mentions that high-performance liquid chromatography (HPLC) was used to measure this compound trough concentrations, suggesting it maintains stability in biological samples for analysis. [] Further research on its stability under different environmental conditions (temperature, humidity, light) would be valuable.
Q6: Does this compound exhibit any catalytic activity itself?
A6: No, this compound is an enzyme inhibitor. It acts by binding to HIV-1 integrase and blocking its catalytic activity, specifically the strand transfer reaction required for integrating viral DNA into the host cell genome. [, ]
Q7: Have any computational studies been conducted on this compound?
A7: Yes, one study used an in vitro-in vivo extrapolation model to predict this compound pharmacokinetics in virtual individuals with different gastrointestinal pH profiles. [] This model successfully predicted key pharmacokinetic variables with good accuracy compared to clinical data, demonstrating the utility of computational approaches in understanding this compound disposition. []
Q8: How do structural modifications of this compound affect its activity?
A8: The provided research focuses primarily on this compound's clinical efficacy and resistance mutations rather than exploring detailed SAR. Investigating the impact of structural changes on this compound's binding affinity, inhibitory potency, and resistance profile would be an interesting avenue for future research.
Q9: What is the primary route of this compound elimination in humans?
A10: this compound is primarily eliminated via metabolism, specifically through glucuronidation by the enzyme UGT1A1. [] This process leads to the formation of this compound glucuronide, the major metabolite, which is then excreted in urine and feces. []
Q10: Does food intake affect this compound pharmacokinetics?
A11: Yes, a study investigating the effect of different meal types on this compound's pharmacokinetic profile found that high-fat meals might influence the drug's absorption and distribution without affecting overall exposure. [] This highlights the importance of understanding food-drug interactions for optimizing this compound therapy.
Q11: How do this compound concentrations vary across different biological compartments?
A12: Research indicates that this compound concentrations can vary significantly between plasma, intracellular compartments (like peripheral blood mononuclear cells), and tissues (such as cervical tissue). [, ] These differences highlight the need to consider tissue-specific drug distribution when evaluating this compound's efficacy and potential for pre-exposure prophylaxis (PrEP). []
Q12: What factors contribute to the high inter-individual variability observed in this compound pharmacokinetics?
A12: Several factors can contribute to variability in this compound's pharmacokinetic profile, including:
- Genetic polymorphisms: Variations in the UGT1A1 gene, particularly the UGT1A16 and 28 alleles, may impact this compound metabolism and clearance. []
- Gastrointestinal factors: Luminal pH and the presence of divalent metals, like magnesium, can influence this compound absorption. [, ]
- Co-administered medications: Drug-drug interactions with medications metabolized by UGT1A1 or those altering gastrointestinal pH can alter this compound exposure. [, , , ]
- Liver transplantation: Patients who have undergone liver transplantation may experience reduced this compound clearance, potentially leading to higher drug exposure. []
Q13: What is the significance of this compound trough concentrations?
A14: this compound's virologic efficacy is best correlated with its trough concentration (C trough). [] Maintaining C trough levels above the 95% inhibitory concentration (IC95) is crucial for achieving optimal viral suppression. [, ]
Q14: How is this compound's efficacy assessed in preclinical settings?
A15: Cell-based assays using TZM-bl indicator cells are used to evaluate this compound's ability to inhibit HIV infection in vitro. [] Additionally, humanized mouse models, such as the humanized BLT (bone marrow-liver-thymus) mice, are valuable tools for studying this compound's antiviral activity, pharmacokinetics, and efficacy in preventing vaginal HIV transmission in vivo. []
Q15: Has this compound's efficacy been demonstrated in clinical trials?
A16: Yes, numerous clinical trials have established the potent and durable antiretroviral activity of this compound in both treatment-naive and treatment-experienced HIV-1-infected patients. [, , , , , , ] When combined with tenofovir/emtricitabine, this compound achieves viral suppression and immune restoration comparable to efavirenz-based regimens. [, ] Moreover, this compound has shown effectiveness in salvage therapy for patients with multidrug-resistant HIV-1 infection. [, , , ]
Q16: What are the primary mechanisms of resistance to this compound?
A17: Resistance to this compound typically arises from mutations in the HIV-1 integrase gene. [, , , ] The most common mutations associated with this compound resistance cluster around three main pathways:
Q17: Does cross-resistance exist between this compound and other integrase inhibitors?
A18: Yes, cross-resistance can occur between this compound and other integrase strand transfer inhibitors (INSTIs), such as elvitegravir and dolutegravir. [, , ] The extent of cross-resistance depends on the specific mutations present in the HIV-1 integrase gene. [, , ] For instance, mutations in the Q148 pathway often confer a higher level of resistance to this compound and elvitegravir compared to dolutegravir. [, ]
Q18: Can this compound resistance impact subsequent treatment options?
A19: Yes, the emergence of this compound resistance mutations can limit future treatment options, particularly with other INSTIs. [, ] Therefore, it's crucial to monitor for resistance development and consider alternative antiretroviral agents when appropriate.
Q19: Does this compound retain any antiviral activity against resistant HIV-1 strains?
A20: Studies suggest that this compound might have limited to no residual antiviral activity against HIV-1 strains harboring major resistance mutations. [, ] This finding raises questions about the benefit of maintaining this compound in salvage regimens for patients with documented resistance. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
![Methyl 3-[[4-(tert-butylamino)-2-methoxyphenyl]sulfamoyl]thiophene-2-carboxylate](/img/structure/B610334.png)

![methyl 4-[3-acetyl-4-hydroxy-1-[2-(2-methyl-1H-indol-3-yl)ethyl]-5-oxo-2H-pyrrol-2-yl]benzoate](/img/structure/B610347.png)