Tétracycline
Vue d'ensemble
Description
La tétracycline est un antibiotique à large spectre appartenant à la famille des tétracyclines, qui a été découverte dans les années 1940. Elle est utilisée pour traiter diverses infections causées par des bactéries, notamment l’acné, le choléra, la brucellose, la peste, le paludisme et la syphilis . La this compound agit en inhibant la synthèse des protéines dans les bactéries, ce qui en fait un agent bactériostatique efficace .
Mécanisme D'action
La tétracycline exerce ses effets en se liant à la sous-unité ribosomale 30S des bactéries, empêchant la fixation de l’aminoacyl-ARNt au ribosome. Cela inhibe la synthèse des protéines, qui est essentielle à la croissance et à la réplication bactériennes. La this compound se lie également à la sous-unité ribosomale 50S dans une moindre mesure, perturbant davantage la synthèse des protéines .
Composés similaires :
- Doxycycline
- Minocycline
- Tigecycline
- Omadacycline
- Eravacycline
Comparaison : La this compound et ses composés similaires partagent un mécanisme d’action commun, mais diffèrent par leurs propriétés pharmacocinétiques et leur spectre d’activité. Par exemple, la doxycycline a une demi-vie plus longue et une meilleure absorption que la this compound, ce qui la rend plus efficace pour certaines infections. La minocycline a une meilleure pénétration dans les tissus, tandis que la tigécycline est efficace contre un éventail plus large de bactéries résistantes .
La this compound reste un antibiotique précieux en raison de son activité à large spectre et de son coût relativement faible. le développement de la résistance a limité son utilisation, ce qui a conduit au développement de nouveaux dérivés de la this compound aux propriétés améliorées .
Applications De Recherche Scientifique
Tetracycline has a wide range of scientific research applications:
Chemistry: Tetracycline is used as a model compound in studies of antibiotic synthesis and degradation.
Biology: It is used to study bacterial resistance mechanisms and the effects of antibiotics on microbial communities.
Medicine: Tetracycline is used to treat bacterial infections and is also studied for its potential use in treating other conditions, such as cancer and inflammatory diseases.
Industry: Tetracycline is used in animal husbandry to promote growth and prevent infections in livestock
Analyse Biochimique
Biochemical Properties
Tetracycline plays a crucial role in biochemical reactions by inhibiting the synthesis of proteins in bacterial cells. It interacts with the 30S ribosomal subunit, preventing the attachment of aminoacyl-tRNA to the mRNA-ribosome complex . This interaction is reversible and leads to a bacteriostatic effect, meaning it stops bacteria from multiplying without necessarily killing them. Tetracycline also binds to the 50S ribosomal subunit to a lesser extent, which may alter the cytoplasmic membrane and cause intracellular components to leak . The compound’s ability to inhibit protein synthesis makes it effective against a wide range of bacterial species.
Cellular Effects
Tetracycline affects various types of cells and cellular processes. In bacterial cells, it inhibits protein synthesis by binding to the 30S ribosomal subunit, which blocks the binding of aminoacyl-tRNA to the ribosome . This inhibition disrupts cell function and prevents the bacteria from growing and reproducing. In eukaryotic cells, tetracycline interacts weakly with the 80S ribosome, leading to a relatively weak inhibition of protein synthesis . This selective inhibition is why tetracycline is effective against bacteria while having limited side effects on human cells.
Molecular Mechanism
The molecular mechanism of tetracycline involves its binding to the 30S ribosomal subunit in bacterial cells. This binding prevents the attachment of aminoacyl-tRNA to the ribosome, thereby inhibiting protein synthesis . Tetracycline’s action is bacteriostatic, meaning it stops bacteria from multiplying without killing them. The compound also binds to the 50S ribosomal subunit to a lesser extent, which may alter the cytoplasmic membrane and cause intracellular components to leak . This dual binding mechanism makes tetracycline a potent inhibitor of bacterial growth.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of tetracycline can change over time. The compound is stable as a dry powder but can degrade in aqueous solutions, especially at higher pH levels . Over time, bacteria can develop resistance to tetracycline, often through the acquisition of genes that code for efflux pumps or ribosomal protection proteins . These resistance mechanisms can reduce the effectiveness of tetracycline in long-term studies. Additionally, tetracycline’s bacteriostatic effect means that its impact on bacterial growth can be observed over several hours to days, depending on the experimental conditions.
Dosage Effects in Animal Models
The effects of tetracycline vary with different dosages in animal models. At therapeutic doses, tetracycline effectively inhibits bacterial growth without causing significant toxicity . At higher doses, tetracycline can cause adverse effects such as gastrointestinal disturbances, hepatotoxicity, and photosensitivity . In some animal models, high doses of tetracycline have been associated with changes in bone and teeth development due to its ability to bind to calcium . These dosage-dependent effects highlight the importance of careful dosing in both clinical and research settings.
Metabolic Pathways
Tetracycline is metabolized in the liver and excreted primarily through the kidneys . The compound undergoes various metabolic transformations, including demethylation, deamination, and hydroxylation . These metabolic pathways are facilitated by enzymes such as cytochrome P450 oxidases. The metabolites of tetracycline can retain some antimicrobial activity, but they are generally less potent than the parent compound . Understanding these metabolic pathways is crucial for optimizing the use of tetracycline in clinical settings.
Transport and Distribution
Tetracycline is transported and distributed within cells and tissues through both passive diffusion and active transport mechanisms . The compound can bind to plasma proteins, which facilitates its distribution throughout the body . Tetracycline is also known to accumulate in certain tissues, such as the liver, kidneys, and bones . This tissue distribution is influenced by factors such as blood flow, tissue permeability, and the presence of binding proteins.
Subcellular Localization
Within cells, tetracycline localizes primarily to the cytoplasm, where it exerts its inhibitory effects on protein synthesis . The compound can also accumulate in the endoplasmic reticulum and other organelles involved in protein synthesis . Tetracycline’s subcellular localization is influenced by its ability to bind to ribosomes and other cellular components. This localization is crucial for its effectiveness as an antibiotic, as it ensures that the compound reaches its target sites within bacterial cells.
Méthodes De Préparation
Voies de synthèse et conditions réactionnelles : La tétracycline peut être synthétisée par diverses méthodes. Une méthode courante implique la fermentation de bactéries Streptomyces, qui produisent naturellement de la this compound. Le processus comprend l’extraction et la purification de l’antibiotique à partir de la culture bactérienne .
Méthodes de production industrielle : Dans les milieux industriels, la this compound est souvent produite de manière semi-synthétique. Le processus commence par la fermentation de bactéries Streptomyces pour produire de la chlorthis compound, qui est ensuite modifiée chimiquement pour produire de la this compound. Cette méthode permet une production à grande échelle de l’antibiotique .
Analyse Des Réactions Chimiques
Types de réactions : La tétracycline subit diverses réactions chimiques, notamment des réactions d’oxydation, de réduction et de substitution. Ces réactions peuvent modifier la structure chimique et les propriétés de la this compound, affectant son efficacité et sa stabilité .
Réactifs et conditions courants :
Oxydation : La this compound peut être oxydée à l’aide de réactifs tels que le peroxyde d’hydrogène ou le permanganate de potassium en conditions acides.
Réduction : La réduction de la this compound peut être obtenue à l’aide de réactifs tels que le borohydrure de sodium ou l’hydrure de lithium et d’aluminium.
Principaux produits formés : Les principaux produits formés à partir de ces réactions comprennent divers dérivés de la this compound, qui peuvent avoir des propriétés pharmacologiques et des applications différentes .
4. Applications de la recherche scientifique
La this compound a un large éventail d’applications de recherche scientifique :
Chimie : La this compound est utilisée comme composé modèle dans les études de synthèse et de dégradation des antibiotiques.
Biologie : Elle est utilisée pour étudier les mécanismes de résistance bactérienne et les effets des antibiotiques sur les communautés microbiennes.
Médecine : La this compound est utilisée pour traiter les infections bactériennes et est également étudiée pour son utilisation potentielle dans le traitement d’autres affections, telles que le cancer et les maladies inflammatoires.
Industrie : La this compound est utilisée dans l’élevage pour favoriser la croissance et prévenir les infections chez le bétail
Comparaison Avec Des Composés Similaires
- Doxycycline
- Minocycline
- Tigecycline
- Omadacycline
- Eravacycline
Comparison: Tetracycline and its similar compounds share a common mechanism of action but differ in their pharmacokinetic properties and spectrum of activity. For example, doxycycline has a longer half-life and better absorption compared to tetracycline, making it more effective for certain infections. Minocycline has better penetration into tissues, while tigecycline is effective against a broader range of resistant bacteria .
Tetracycline remains a valuable antibiotic due to its broad-spectrum activity and relatively low cost. the development of resistance has limited its use, prompting the development of newer tetracycline derivatives with improved properties .
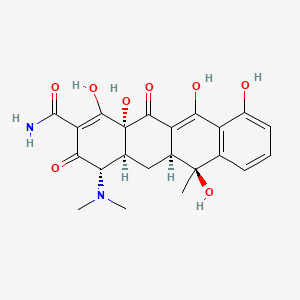
Propriétés
IUPAC Name |
4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25-26,29,31-32H,7H2,1-3H3,(H2,23,30) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NWXMGUDVXFXRIG-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1(C2CC3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H24N2O8 | |
| Details | Computed by PubChem 2.2 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
444.4 g/mol | |
| Details | Computed by PubChem 2.2 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
60-54-8 | |
| Record name | tetracycline | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=108579 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
Q1: How does tetracycline interact with its target and what are the downstream effects?
A1: Tetracycline exerts its antibacterial activity by binding to the bacterial ribosome, specifically at the 30S ribosomal subunit [, ]. This binding interferes with the binding of aminoacyl-tRNA to the ribosomal acceptor (A) site, thereby inhibiting protein synthesis and halting bacterial growth [, ].
Q2: Can you provide some structural characterization of tetracycline?
A2: Tetracycline is a yellow, crystalline compound with the molecular formula C22H24N2O8 and a molecular weight of 444.44 g/mol. The molecule features a tetracyclic naphtacene carboxamide ring system [, ]. Spectroscopic studies, including FT-IR and UV-Vis, have been employed to characterize the compound and investigate potential changes in its structural properties upon various treatments [].
Q3: How does the structure of tetracycline relate to its activity?
A3: The carboxamide group at the C2 position of the tetracycline molecule is essential for its transport into bacterial cells []. Modifications to the tetracycline structure can significantly alter its activity, potency, and selectivity [].
Q4: Are there any studies on the stability of tetracycline?
A4: Yes, studies have explored the stability of tetracycline hydrochloride under various conditions. One study investigated the effect of biofield treatment on chloramphenicol and tetracycline hydrochloride using FT-IR spectroscopy, suggesting that the treatment might enhance the chemical stability of both drugs []. Another study examined the release kinetics of 25% tetracycline hydrochloride-loaded ethylene vinyl acetate fibers, revealing a considerable decrease in the amount of biologically active tetracycline remaining in the fibers over time [].
Q5: Are there alternative compounds to tetracycline?
A5: Yes, other antibiotics, like macrolides and quinolones, can be used as alternatives to tetracycline []. The choice of treatment depends on factors such as the specific bacterial infection, resistance patterns, and patient-specific considerations [, ].
Q6: What analytical methods are employed to study tetracycline?
A6: Various analytical methods are used to characterize, quantify, and monitor tetracycline. These include:
- High-Performance Liquid Chromatography (HPLC): This method is commonly used to separate and quantify tetracycline and its analogs in various matrices, including honey, milk, and pharmaceutical formulations [, , , ].
- Liquid Chromatography-Mass Spectrometry (LC-MS/MS): This technique offers high sensitivity and selectivity for the determination of tetracycline residues in complex matrices like honey [].
- Ultra-Performance Liquid Chromatography (UPLC): This method provides rapid and efficient separation of tetracycline for analysis, as demonstrated in a study evaluating water-flow variation and tetracycline hydrochloride administration in swine [].
- Spectrophotometry: This method, often coupled with derivatization techniques like diazotization, can be used to quantify tetracycline hydrochloride in pharmaceutical formulations [].
- Agar Diffusion Inhibition Assay: This microbiological method assesses the biological activity of tetracycline, for example, in drug-loaded fibers [].
Q7: What are the mechanisms of resistance to tetracycline?
A7: Bacteria can develop resistance to tetracycline through several mechanisms:
- Efflux Pumps: Bacteria can acquire genes encoding efflux pumps, such as TetA(P) from Clostridium perfringens, which actively transport tetracycline out of the cell, reducing its intracellular concentration [].
- Ribosome Protection Proteins: These proteins, like TetM and TetO, can bind to the ribosome and displace tetracycline from its binding site, allowing protein synthesis to continue [].
- Mutations in Ribosomal RNA: Mutations in the 16S rRNA genes, particularly in the primary tetracycline binding pocket (Tet-1 site), can reduce the binding affinity of tetracycline to the ribosome, conferring decreased susceptibility [].
Q8: How prevalent is tetracycline resistance?
A8: The prevalence of tetracycline resistance varies depending on the bacterial species, geographic location, and antibiotic usage patterns. Studies have reported a high prevalence of tetracycline resistance genes, such as tetA and tetB, in Escherichia coli isolates from broiler chickens []. Additionally, resistance to tetracycline is a concern in Staphylococcus aureus [].
Q9: Are there any strategies to overcome tetracycline resistance?
A9: Researchers are exploring various strategies to combat tetracycline resistance:
- Combination Therapy: Using tetracycline in combination with other antibiotics, such as rifampicin, can enhance its activity against resistant strains [].
- Drug Delivery Systems: Novel drug delivery systems, such as encapsulation in cyclodextrins, are being explored to improve the bioavailability and efficacy of tetracycline [].
Q10: What is the environmental impact of tetracycline?
A10: Tetracycline, like other antibiotics, can enter the environment through wastewater and agricultural runoff, potentially contributing to the spread of antibiotic resistance genes []. Studies have investigated the use of UV light disinfection to degrade tetracycline in wastewater effluents []. Other research focuses on using porous graphitic biochar for the adsorption and removal of tetracycline from aqueous solutions [].
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.