Tetracycline
説明
テトラサイクリンは、1940 年代に発見されたテトラサイクリン系に属する広域スペクトル抗生物質です。 ニキビ、コレラ、ブルセラ症、ペスト、マラリア、梅毒など、細菌が原因のさまざまな感染症の治療に使用されます 。 テトラサイクリンは細菌におけるタンパク質合成を阻害することで作用し、効果的な静菌剤となります .
2. 製法
合成経路と反応条件: テトラサイクリンはさまざまな方法で合成できます。一般的な方法の 1 つには、テトラサイクリンを自然に生成するストレプトマイセス属細菌の発酵が含まれます。 このプロセスには、細菌培養液からの抗生物質の抽出と精製が含まれます .
工業生産方法: 工業的な環境では、テトラサイクリンはしばしば半合成的に生産されます。プロセスは、ストレプトマイセス属細菌を発酵させてクロルテトラサイクリンを生成することから始まり、その後、化学的に修飾してテトラサイクリンを生成します。 この方法は、抗生物質の大規模生産を可能にします .
作用機序
テトラサイクリンは、細菌の 30S リボソームサブユニットに結合することで効果を発揮し、アミノアシルtRNAがリボソームに結合するのを防ぎます。これは、細菌の増殖と複製に不可欠なタンパク質合成を阻害します。 テトラサイクリンは、50S リボソームサブユニットにも結合しますが、その程度は小さく、タンパク質合成をさらに阻害します .
類似化合物:
- ドキシサイクリン
- ミノサイクリン
- チゲサイクリン
- オマダサイクリン
- エラバサイクリン
比較: テトラサイクリンとその類似化合物は、共通の作用機序を共有していますが、薬物動態特性と活性スペクトルが異なります。たとえば、ドキシサイクリンはテトラサイクリンと比較して半減期が長く、吸収が優れているため、特定の感染症に対してより効果的です。 ミノサイクリンは組織への浸透性が高く、チゲサイクリンは幅広い耐性菌に対して効果的です .
テトラサイクリンは、広域スペクトル活性と比較的低価格であるため、貴重な抗生物質であり続けています。 耐性菌の出現により、その使用は制限されてきましたが、より優れた特性を持つ新しいテトラサイクリン誘導体の開発が促されました .
生化学分析
Biochemical Properties
Tetracycline plays a crucial role in biochemical reactions by inhibiting the synthesis of proteins in bacterial cells. It interacts with the 30S ribosomal subunit, preventing the attachment of aminoacyl-tRNA to the mRNA-ribosome complex . This interaction is reversible and leads to a bacteriostatic effect, meaning it stops bacteria from multiplying without necessarily killing them. This compound also binds to the 50S ribosomal subunit to a lesser extent, which may alter the cytoplasmic membrane and cause intracellular components to leak . The compound’s ability to inhibit protein synthesis makes it effective against a wide range of bacterial species.
Cellular Effects
This compound affects various types of cells and cellular processes. In bacterial cells, it inhibits protein synthesis by binding to the 30S ribosomal subunit, which blocks the binding of aminoacyl-tRNA to the ribosome . This inhibition disrupts cell function and prevents the bacteria from growing and reproducing. In eukaryotic cells, this compound interacts weakly with the 80S ribosome, leading to a relatively weak inhibition of protein synthesis . This selective inhibition is why this compound is effective against bacteria while having limited side effects on human cells.
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the 30S ribosomal subunit in bacterial cells. This binding prevents the attachment of aminoacyl-tRNA to the ribosome, thereby inhibiting protein synthesis . This compound’s action is bacteriostatic, meaning it stops bacteria from multiplying without killing them. The compound also binds to the 50S ribosomal subunit to a lesser extent, which may alter the cytoplasmic membrane and cause intracellular components to leak . This dual binding mechanism makes this compound a potent inhibitor of bacterial growth.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound can change over time. The compound is stable as a dry powder but can degrade in aqueous solutions, especially at higher pH levels . Over time, bacteria can develop resistance to this compound, often through the acquisition of genes that code for efflux pumps or ribosomal protection proteins . These resistance mechanisms can reduce the effectiveness of this compound in long-term studies. Additionally, this compound’s bacteriostatic effect means that its impact on bacterial growth can be observed over several hours to days, depending on the experimental conditions.
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At therapeutic doses, this compound effectively inhibits bacterial growth without causing significant toxicity . At higher doses, this compound can cause adverse effects such as gastrointestinal disturbances, hepatotoxicity, and photosensitivity . In some animal models, high doses of this compound have been associated with changes in bone and teeth development due to its ability to bind to calcium . These dosage-dependent effects highlight the importance of careful dosing in both clinical and research settings.
Metabolic Pathways
This compound is metabolized in the liver and excreted primarily through the kidneys . The compound undergoes various metabolic transformations, including demethylation, deamination, and hydroxylation . These metabolic pathways are facilitated by enzymes such as cytochrome P450 oxidases. The metabolites of this compound can retain some antimicrobial activity, but they are generally less potent than the parent compound . Understanding these metabolic pathways is crucial for optimizing the use of this compound in clinical settings.
Transport and Distribution
This compound is transported and distributed within cells and tissues through both passive diffusion and active transport mechanisms . The compound can bind to plasma proteins, which facilitates its distribution throughout the body . This compound is also known to accumulate in certain tissues, such as the liver, kidneys, and bones . This tissue distribution is influenced by factors such as blood flow, tissue permeability, and the presence of binding proteins.
Subcellular Localization
Within cells, this compound localizes primarily to the cytoplasm, where it exerts its inhibitory effects on protein synthesis . The compound can also accumulate in the endoplasmic reticulum and other organelles involved in protein synthesis . This compound’s subcellular localization is influenced by its ability to bind to ribosomes and other cellular components. This localization is crucial for its effectiveness as an antibiotic, as it ensures that the compound reaches its target sites within bacterial cells.
準備方法
Synthetic Routes and Reaction Conditions: Tetracycline can be synthesized through various methods. One common method involves the fermentation of Streptomyces bacteria, which naturally produce this compound. The process includes the extraction and purification of the antibiotic from the bacterial culture .
Industrial Production Methods: In industrial settings, this compound is often produced semi-synthetically. The process begins with the fermentation of Streptomyces bacteria to produce chlorthis compound, which is then chemically modified to produce this compound. This method allows for large-scale production of the antibiotic .
化学反応の分析
反応の種類: テトラサイクリンは、酸化反応、還元反応、置換反応など、さまざまな化学反応を起こします。 これらの反応は、テトラサイクリンの化学構造と特性を変更し、その効力と安定性に影響を与える可能性があります .
一般的な試薬と条件:
酸化: テトラサイクリンは、過酸化水素や過マンガン酸カリウムなどの試薬を使用して、酸性条件下で酸化することができます。
還元: テトラサイクリンの還元は、水素化ホウ素ナトリウムや水素化リチウムアルミニウムなどの試薬を使用して達成できます。
置換: 置換反応は、多くの場合、ハロゲンやアルキル化剤などの試薬を使用して、テトラサイクリン分子の官能基を置換することを含みます.
生成される主な生成物: これらの反応から生成される主な生成物には、さまざまなテトラサイクリン誘導体が含まれ、これらは異なる薬理学的特性と用途を持つ可能性があります .
4. 科学研究への応用
テトラサイクリンは、幅広い科学研究用途を持っています。
化学: テトラサイクリンは、抗生物質の合成と分解の研究におけるモデル化合物として使用されます。
生物学: 細菌の耐性メカニズムと、抗生物質が微生物群集に与える影響を研究するために使用されます。
医学: テトラサイクリンは、細菌感染症の治療に使用され、癌や炎症性疾患などの他の状態の治療における潜在的な用途についても研究されています。
科学的研究の応用
Tetracycline has a wide range of scientific research applications:
Chemistry: this compound is used as a model compound in studies of antibiotic synthesis and degradation.
Biology: It is used to study bacterial resistance mechanisms and the effects of antibiotics on microbial communities.
Medicine: this compound is used to treat bacterial infections and is also studied for its potential use in treating other conditions, such as cancer and inflammatory diseases.
Industry: this compound is used in animal husbandry to promote growth and prevent infections in livestock
類似化合物との比較
- Doxycycline
- Minocycline
- Tigecycline
- Omadacycline
- Eravacycline
Comparison: Tetracycline and its similar compounds share a common mechanism of action but differ in their pharmacokinetic properties and spectrum of activity. For example, doxycycline has a longer half-life and better absorption compared to this compound, making it more effective for certain infections. Minocycline has better penetration into tissues, while tigecycline is effective against a broader range of resistant bacteria .
This compound remains a valuable antibiotic due to its broad-spectrum activity and relatively low cost. the development of resistance has limited its use, prompting the development of newer this compound derivatives with improved properties .
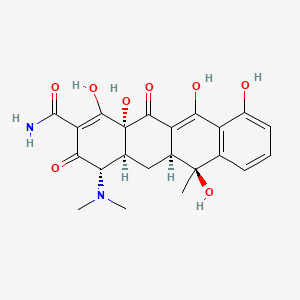
特性
IUPAC Name |
4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25-26,29,31-32H,7H2,1-3H3,(H2,23,30) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NWXMGUDVXFXRIG-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1(C2CC3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H24N2O8 | |
| Details | Computed by PubChem 2.2 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
444.4 g/mol | |
| Details | Computed by PubChem 2.2 (PubChem release 2021.10.14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
60-54-8 | |
| Record name | tetracycline | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=108579 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
Q1: How does tetracycline interact with its target and what are the downstream effects?
A1: this compound exerts its antibacterial activity by binding to the bacterial ribosome, specifically at the 30S ribosomal subunit [, ]. This binding interferes with the binding of aminoacyl-tRNA to the ribosomal acceptor (A) site, thereby inhibiting protein synthesis and halting bacterial growth [, ].
Q2: Can you provide some structural characterization of this compound?
A2: this compound is a yellow, crystalline compound with the molecular formula C22H24N2O8 and a molecular weight of 444.44 g/mol. The molecule features a tetracyclic naphtacene carboxamide ring system [, ]. Spectroscopic studies, including FT-IR and UV-Vis, have been employed to characterize the compound and investigate potential changes in its structural properties upon various treatments [].
Q3: How does the structure of this compound relate to its activity?
A3: The carboxamide group at the C2 position of the this compound molecule is essential for its transport into bacterial cells []. Modifications to the this compound structure can significantly alter its activity, potency, and selectivity [].
Q4: Are there any studies on the stability of this compound?
A4: Yes, studies have explored the stability of this compound hydrochloride under various conditions. One study investigated the effect of biofield treatment on chloramphenicol and this compound hydrochloride using FT-IR spectroscopy, suggesting that the treatment might enhance the chemical stability of both drugs []. Another study examined the release kinetics of 25% this compound hydrochloride-loaded ethylene vinyl acetate fibers, revealing a considerable decrease in the amount of biologically active this compound remaining in the fibers over time [].
Q5: Are there alternative compounds to this compound?
A5: Yes, other antibiotics, like macrolides and quinolones, can be used as alternatives to this compound []. The choice of treatment depends on factors such as the specific bacterial infection, resistance patterns, and patient-specific considerations [, ].
Q6: What analytical methods are employed to study this compound?
A6: Various analytical methods are used to characterize, quantify, and monitor this compound. These include:
- High-Performance Liquid Chromatography (HPLC): This method is commonly used to separate and quantify this compound and its analogs in various matrices, including honey, milk, and pharmaceutical formulations [, , , ].
- Liquid Chromatography-Mass Spectrometry (LC-MS/MS): This technique offers high sensitivity and selectivity for the determination of this compound residues in complex matrices like honey [].
- Ultra-Performance Liquid Chromatography (UPLC): This method provides rapid and efficient separation of this compound for analysis, as demonstrated in a study evaluating water-flow variation and this compound hydrochloride administration in swine [].
- Spectrophotometry: This method, often coupled with derivatization techniques like diazotization, can be used to quantify this compound hydrochloride in pharmaceutical formulations [].
- Agar Diffusion Inhibition Assay: This microbiological method assesses the biological activity of this compound, for example, in drug-loaded fibers [].
Q7: What are the mechanisms of resistance to this compound?
A7: Bacteria can develop resistance to this compound through several mechanisms:
- Efflux Pumps: Bacteria can acquire genes encoding efflux pumps, such as TetA(P) from Clostridium perfringens, which actively transport this compound out of the cell, reducing its intracellular concentration [].
- Ribosome Protection Proteins: These proteins, like TetM and TetO, can bind to the ribosome and displace this compound from its binding site, allowing protein synthesis to continue [].
- Mutations in Ribosomal RNA: Mutations in the 16S rRNA genes, particularly in the primary this compound binding pocket (Tet-1 site), can reduce the binding affinity of this compound to the ribosome, conferring decreased susceptibility [].
Q8: How prevalent is this compound resistance?
A8: The prevalence of this compound resistance varies depending on the bacterial species, geographic location, and antibiotic usage patterns. Studies have reported a high prevalence of this compound resistance genes, such as tetA and tetB, in Escherichia coli isolates from broiler chickens []. Additionally, resistance to this compound is a concern in Staphylococcus aureus [].
Q9: Are there any strategies to overcome this compound resistance?
A9: Researchers are exploring various strategies to combat this compound resistance:
- Combination Therapy: Using this compound in combination with other antibiotics, such as rifampicin, can enhance its activity against resistant strains [].
- Drug Delivery Systems: Novel drug delivery systems, such as encapsulation in cyclodextrins, are being explored to improve the bioavailability and efficacy of this compound [].
Q10: What is the environmental impact of this compound?
A10: this compound, like other antibiotics, can enter the environment through wastewater and agricultural runoff, potentially contributing to the spread of antibiotic resistance genes []. Studies have investigated the use of UV light disinfection to degrade this compound in wastewater effluents []. Other research focuses on using porous graphitic biochar for the adsorption and removal of this compound from aqueous solutions [].
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。